Correlation of coronary plaque characteristics and obstructive stenosis with chronic kidney disease by coronary CT angiography
Introduction
Chronic kidney disease (CKD) has been a worldwide public health problem (1-4). Increasing prevalence of CKD is astounding. In the United States about 8 million adults had CKD of at least stage 3 (1), and investigation on adult population of Beijing in China showed that the prevalence of CKD was 13.0% (2). As a result, CKD has been an independent risk factor for adverse cardiovascular disease (CVD) (3-5). Progressive impairment of renal function will independently give rise to increased risks of CVD. CKD patients are more likely to die of CVD than to progress to ESKD (6). Epidemiological studies from China showed the prevalence of ESKD in China is far less than the prevalence of mild or moderate renal insufficiency (2,7,8). Hence, the burden of CVD in the population with CKD should be emphasized. It may induce a greater impairment of health than does ESKD.
In the other words, CKD also accelerate the occurrence of coronary artery disease (CAD). Date from epidemiologic studies reported that glomerular filtration rates (GFR) is negatively correlated with CAD (3,5). CAD has long been identified as a leading cause of death among patients with ESRD. Decreased GFR and proteinuria are both found to be independently associated with CAD. Considering the high risk of CVD in the population with CKD, the American College of Cardiology/American Heart Association task force and the National Kidney Foundation in 2003 regarded CKD as a CAD risk equivalent (9).
Recently, the development of computed tomographic has almost solved the problem of cardiac examination. Coronary computed tomographic angiography (CCTA) has been playing a significant role in the noninvasive assessment of coronary plaque and stenosis. It not only leads to higher diagnostic accuracy for CAD, but also reduces the dose of contrast agent and radiation. There was increasing evidence proves that CCTA has high diagnostic accuracy for CAD (10,11). Although several studies have found the negative correlation of CKD with CAD, the coronary plaque characteristics of patients with CKD are still remains unclear. Therefore, this present study aimed to investigate the correlation of coronary plaque characteristics and obstructive stenosis with CKD in Dual source computed tomography (DSCT).
Methods
Study population
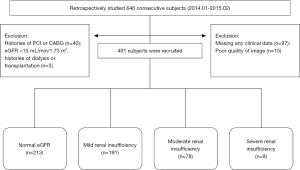
We retrospectively studied 646 consecutive subjects who were suspected CAD undergoing CCTA with DSCT from March 2014 to February 2015 in the Department of Imaging Center of Nanfang Hospital. Firstly, 151 subjects were excluded: missing any data of clinical information (n=97), had histories of percutaneous coronary intervention (PCI, n=32) or coronary artery by-pass graft (CABG, n=8), poor quality of image (n=15), patients with eGFR <15 mL/min/1.73 m2 and/or performed dialysis or transplantation (n=3). As a result, 491 subjects [345 males and 146 females, age 26-87 years, mean age (59.0±10.6) years] were enrolled in our study. The process was showed in Figure 1.
Collection of clinical characteristics
The lifestyle and clinical characteristics of all subjects were obtained by referred to completion of electronic medical record or accomplished an interview. We collected information including age, sex, height (cm), body weight (kg), serum creatinine (Scr), blood pressure (BP), smoking status, serum levels of triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), fasting blood glucose, medical history of hypertension (HT), diabetes mellitus (DM) and hyperlipidemia (HPL). Evaluation of renal function was based on the estimated GFR (eGFR). For all participants the eGFR were calculated by the modification of diet in renal disease (MDRD) study Eq. [1] (12):
eGFR (mL/min/1.73 m2) =186× (SCr/88.41)−1.154× (age)−0.203 (0.742 if female) [1]
Scr was tested in the first month before undergoing CCTA with extracting fasting venous blood. The stage of CKD based on the K/DOQI (13) clinical practice guidelines for CKD as follows: CKD stage 1, renal function is normal or with increased GFR (eGFR ≥90 mL/min/1.73 m2); CKD stage 2, renal function is mildly insufficient (eGFR <89 mL/min/1.73 m2, ≥60 mL/min/1.73 m2); CKD stage 3, renal function is moderately insufficient (eGFR<60 mL/min/1.73 m2, ≥30 mL/min/1.73 m2); CKD stage 4, renal function is severely insufficient (eGFR <30 mL/min/1.73 m2, ≥15 mL/min/1.73 m2); CKD stage 5, kidney failure (eGFR <15 mL/min/1.73 m2). Afterwards, we divided all subjects into four categories: normal eGFR group (eGFR ≥90 mL/min/1.73 m2), mild renal insufficiency group (eGFR <89 mL/min/1.73 m2, ≥60 mL/min/1.73 m2), moderate renal insufficiency group(eGFR <60 mL/min/1.73 m2, ≥30 mL/min/1.73 m2), and severe renal insufficiency group (eGFR <30 mL/min/1.73 m2, ≥15 mL/min/1.73 m2). HT was defined as a self-reported history of HT or treatment of antihypertensive treatment, or blood pressure ≥140/90 mmHg. HPL was defined as self-reported history of HPL or a lipid-lowing treatment, or LDL-C >140 mg/dL or TG >200 mg/dL. DM was defined as a self-reported history of DM or a glucose-lowing treatment. Body mass index (BMI) was calculated by dividing body weight (kg) by the square of height (m).
CCTA scan protocol
The study was approved by the ethical committee, and obtained patient himself or her family’s agreement and signed the medical informed consent document. All patients were performed with CCTA using ECG-gated DSCT (Somatom Definition, Siemens Healthcare, Forchheim, Germany). Before examination, blood pressure and heart rate were measured, breath training was did, 20 G trocar was leaved in patient’s median cubital vein, 1-2 snap Nitroglycerin was injected in patient sublingual mucosal. At the beginning, coronary calcium scoring (CCS) scan was performed; scan range was from tracheal juga to the diaphragm. Then, 75-85 mL of contrast medium (Iopamiron 370, Bayer Schering Pharma AG, Berlin, Germany), according to the patient’s weight, was injected through a dual channel high pressure syringe at a rate of 5.0<5.5 mL/s into cubital vein, followed by 30 mL of saline solution chaser, using a bolus tracking technique at the slice of aortic root to determine the trigger time. When the density reached a predefined threshold of 90 Hounsfield units (HU), the scan automatically started with a 6 seconds scan delay during one breath-hold with simultaneous recording of the ECG-tracing. Heart rate and ECG were monitored during CCTA. The image parameters were a slice collimation of 32 mm × 0.6 mm, slice acquisition 64 mm × 0.6 mm by means of a Z-axis flying focal spot, 120 KV, 350 mAs, 0.33 s rotation time. All patients were performed with retrospective ECG gated scan.
Image reconstruction
Original date (standard DICOM 3.0 image) was transmitted to the Syngo post-processing workstation of Siemens, layer thickness of 0.75 mm, interval of 1 mm. Two independent blinded radiologists analysed the coronary lesion with Circulation software, and measured the CCS with CaScoring software. Before analysis, we would select one of the best phase for reconstruction by means of volume rending (VR), curved planar reformation (CPR), maximum intensive projection (MIP), multiple planar reconstruction techniques (MPR) and virtual digital subtraction angiography (DSA). As soon as reconstruction was accomplished, all images would be sent to picture achieving and communication system (PACS) of Nanfang Hospital for diagnosis.
Image analysis
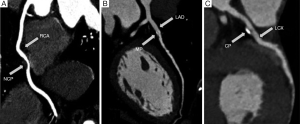
All images would be analyzed by two independent blinded radiologists in PACS. If views inconsistent, carefully discussion would be processed for the uniform views. Coronary image quality were divided into 4 levels according to the 4-point scale as follow: 1, excellent, no motion artifacts, clear delineation of vascular contours; 2, good, minor artifacts, mild blurring of vascular contours; 3, adequate, moderate artifacts, moderate blurring without structure discontinuity of vessel; 4, not evaluative, doubling or discontinuity in the course of the segment preventing evaluation or vessel structures not differentiable (14). Score 1-3 were considered for diagnostic, score 4 was excluded. Based on the recommendations of the American Heart Association study, coronary arteries were divided into 15 segments and 4 main branches (15). CCS was calculated by the Agatston score (16), calcification was defined as a threshold of 130 HU of attenuation value and 1 mm2 of Region of Interest Controls (ROI) area. CCS =[calcified plaque (CP) area] × (highest CT value coefficient), coefficient of HU: 1=133-199 HU; 2=200-299 HU; 3=300-399 HU; 4≥400 HU. Plaque was clarified into 3 types: CP, mixed plaque (MP), non-calcified plaque (NCP). Calcified plaque was defined as a plague with higher CT value than vessel, non-calcified plaque was defined as a plaque with lower CT value than vessel, MP was defined as a plaque consist of calcium and soft tissue ingredient. Obstructive stenosis was described as follows: no stenosis or existence of irregular vascular contours (stenosis percentage <30%), mild stenosis (stenosis percentage ≥30%, <50%), moderate stenosis (stenosis percentage ≥50%, <75%), severe stenosis or blocking (stenosis percentage ≥75%). Multi-vessels disease was defined as two or more vessels prevalence of any plaque or stenosis (Figure 2).
Statistic analysis
Continuous variables of clinical characteristic were presented as mean and standard deviations (mean±SD). Regular smoking, prevalence of HT, HPL, DM were categorized as yes/no. Categorical variables of clinical characteristic were presented as percentage which was the frequency of positive events. Comparison of continuous variables was performed with one-way ANOVA and least-significant difference (LSD) was used for multiple comparisons. Comparison of categorical variables was performed with chi-square test. Firstly, Spearman’s correlation was applied to evaluate the correlation of coronary lesion with CKD. Then, partial correlation was used for adjustment for following covariates: age, sex, regular smoking, BMI, prevalence of HT, HPL, and DM. All P values were 2-sided, P values <0.05 was considered to identify statistically significant differences. All analysis was carried out using SPSS, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline clinical characteristics
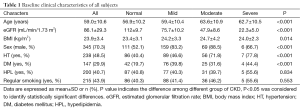
All images were of sufficient quality for analysis. After examination of CCTA, all the subjects have not appeared the exacerbation of renal function during our follow-up for three to 6 month. A total of 491 subjects were divided into four groups: normal eGFR group (n=213), mild renal insufficiency group (n=191) moderate renal insufficiency group (n=78), severe renal insufficiency group (n=9). The baseline clinical characteristics were showed in Table 1: among all subjects the mean eGFR was 86.1±29.3, normal renal insufficiency group was 112±19.7, mild renal insufficiency group was 75.7±10.2, moderate renal insufficiency group was 47.9±8.6, and severe renal insufficiency group was 22.3±5, difference among groups was statistically significant (P<0.0001). Compared with normal eGFR group, mild renal insufficiency, moderate renal insufficiency group and severe renal insufficiency group had a higher prevalence of advanced age, BMI, male, HT, and DM, differences among groups all were statistically significant (P<0.05). However, differences among groups of prevalence of HPL and regular smoking were no statistically significant (P=0.834, P=0.553).
Full table
Coronary artery disease (CAD)
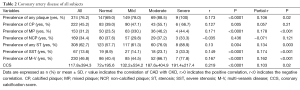
The results were showed in Table 2.
Full table
Coronary plaque characteristics
Coronary plaque was found in 374 of 491 subjects (76.2%). Prevalence of any plaque, calcified plaque and MP were positively correlate with CKD (r=0.173, P<0.0001; r=0.127, P=0.005; r=0.171, P<0.0001). Prevalence of non-calcified plaque had no correlation with CKD (r=−0.035, P=0.436). After adjustment for traditional risk factors, prevalence of any plaque and MP were still positively correlate with CKD (r=0.106, P=002; r=0.178, P<0.0001). Nevertheless, prevalence of calcified plaque being no correlation with CKD.
Obstructive stenosis
Stenosis was found in 308 of 491 subjects (62.7%). Prevalence of any stenosis and severe stenosis were positively correlate with CKD (r=0.13, P<0.001; r=0.149, P<0.001). After adjustment for traditional cardiovascular risk factors, prevalence of any stenosis and severe stenosis were still positively correlate with CKD (r=0.134, P=0.003; r=0.174, P<0.001)
Prevalence of multi-vessels disease and CCS
Multi-vessels disease was found 230 of 491 subjects (46.8%). Prevalence of multi-vessels lesion was positively correlate with CKD (r=0.167, P<0.001). After adjustment for traditional cardiovascular risk factors, prevalence of multi-vessels lesion was still positively correlate with CKD (r=0.167, P<0.001). CCS of all subjects was 117.8±394.3, normal eGFR group was 72±195.6, mild renal insufficiency group was 132.2±534.2, moderate insufficiency group was 187.6±404.9, severe renal insufficiency group was 191.4±217.4. CCS was positively correlate with the CKD (r=0.219, P<0.001). After adjustment for traditional cardiovascular risk factors, CCS were still positively correlate with CKD (r=0.103, P=0.02).
Discussion
CKD is characterized by a progressive decline in GFR over many years resulting in permanent kidney failure requiring dialysis or transplantation. Although the mechanisms linking renal insufficiency to high CAD risk have not been completely understood and further study should be did, publics had an indisputable implications of the adverse result for health. Previous studies reported that both traditional risk factors (advanced age, male sex, DM, HT, HPL, smoking, obesity, sedentary lifestyle, and family history of CVD and so on) and nontraditional risk factors (endothelial dysfunction, CKD-MBD abnormalities, increased oxidative stress, and inflammation and so on) commonly contributed to the formation of coronary plaques and heavy burden of CVD to the patients with renal insufficiency. Traditional cardiovascular risk factors were reliable predictor for cardiovascular event, especially for CAD (17-21). The present study focused attentions on the traditional cardiovascular risk factors, the results showed that population with renal insufficiency had a higher prevalence of advanced age, advanced BMI, male, HT, and DM. Though, prevalence of HPL and regular smoking had no differences among different groups in the present study, analysis of risk factor of CKD in previous studies reported that total cholesterol level in patients with CKD was higher than patients with no CKD (5,17). Hence, the results indicate that patients with CKD easily exposed to traditional cardiovascular risk factors. As a disappointed consequence, it will give rise to higher incidence of CVD.
Previous studies demonstrated that moderate renal dysfunction may accelerate the formation of coronary plague and increase the frequency of plaque disruption. Moreover, the morphology of atherosclerotic plaque differs between patients with and without CKD (22-25). Our study recruited subjects with normal eGFR to severe renal insufficiency to discuss the correlation with the coronary plaque characteristics and obstructive stenosis. To the best of our knowledge, few studies recruited different degree of renal insufficiency to investigate the correlation mentioned above, especially patients with an eGFR <30 mL/min/1.73 m2. We demonstrated that the correlation of prevalence of any coronary plaque, MP, any stenosis, severe stenosis, and multi-vessels disease were significant positively correlate with different degree of renal insufficiency. In addition, after adjustment for traditional cardiovascular risk factors, the relationship still exists. It means that the exacerbation of renal function will accompany with higher risk of prevalence of coronary plaques and obstructive stenosis. Even mild renal insufficiency will be a risk factor of CAD. The existence of vulnerable plaques aggrandizes the risk of suffering acute coronary syndrome (ACS). Although CCTA can’t reliably distinct the vulnerable plaques, it can accurately distinct the composition of coronary plaque. The study did by Pundziute et al. showed that the vulnerable plaques usual be mixed-plaque (26). In the present study, higher prevalence of MP indicates us that heavier impairment of renal function may motivate higher risk of ACS. Although the correlation of prevalence of calcified plaque with renal insufficiency had no statistically significance, the CACS was positively correlate with the renal insufficiency after adjustment for traditional risk factors. It is well known that vascular calcification is a dependable predictor for adverse cardiovascular events. Quantification of coronary arterial calcification (CAC) provides prognostic information beyond identification of traditional CV risk factors (27,28). Higher CCS was associated with higher prevalence, more diffuse and greater extent of CAD in patients with renal insufficiency (29).
In our present study, less than 1/5 subjects with moderate or severe renal insufficiency, but of them less than 1/3 suffered from coronary severe stenosis. Moreover, more than 1/2 of CKD patients with mild or no CAD. Hence, CCTA could be one of the useful methods for screening of CKD patients who were suspected CAD. It is important to noninvasively identify such low-risk patients for CAD in the high-risk group to spare them further invasive examinations. These results provided further supporting evidence for the relationship between elevated cardiovascular risk and different degree of CKD by CCTA. We suppose that one of the mechanisms by which CKD is associated with a high risk of CVD would be through the different coronary plaques. Accordingly, a reasonable treatment to reduce the risk in these patients would prevent the formation of plaques or stabilize them aggressively. Early detection and treatment of renal insufficiency may reduce the societal burden of CAD, as well as CVD. As a long-term results, it is significant for reduce cardiovascular morbidity and mortality. Although risk factor modification in CKD patients can substantially decrease cardiovascular morbidity and mortality based on the data presented, the presence of CKD should prompt aggressive measures to reach goals for blood pressure and cholesterol recommended for the highest cardiovascular risk group.
Our study had following limitation. Firstly, the patient population of this present study was a relatively small sample, especially the patients with severe renal insufficiency (n=9), because of the risk of contrast induced nephropathy. All the subjects recruited who were suspected CAD undergoing CCTA in a solo health community. As a result, it cannot represent the general population and most of the patients could accompany with coronary heart disease risk factors. Therefore, selection bias could influence the results, and information about causation could not be provided. Secondly, we measured the serum creatinine only one time and we measured the GFR with MDRD equation. The effect of variation of serum creatinine cannot be excluded. MADR equation is not the most accurate measurement of GFR, the result of MADR equation is an estimate GFR, and hence, the measurement error would influence the results. Thirdly, we did not have information regarding proteinuria and albuminuria because we did not collect urine samples. Although CCTA is a well-established imaging technique for detection of coronary plaques, the functional relevance of the plaques remains unsubstantiated. Finally, we have not investigated patient outcomes because of the relatively short follow-up time.
Conclusions
All in all, more serious renal insufficiency will indicate higher risk of coronary plaque, MPs, obstructive stenosis, multi-vessels disease and CCS. CKD patients from mild renal insufficiency to severe renal insufficiency are the risk factors for CAD. CKD is closely related with the occurrence of CAD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003;41:1-12. [PubMed]
- Zhang L, Zhang P, Wang F, et al. Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis 2008;51:373-84. [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [PubMed]
- Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004;164:659-63. [PubMed]
- Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol 2002;13:745-53. [PubMed]
- Pun PH, Smarz TR, Honeycutt EF, et al. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int 2009;76:652-8. [PubMed]
- Li ZY, Xu GB, Xia TA, et al. Prevalence of chronic kidney disease in a middle and old-aged population of Beijing. Clin Chim Acta 2006;366:209-15. [PubMed]
- Liu BC, Wu XC, Wang YL, et al. Investigation of the prevalence of CKD in 13,383 Chinese hospitalised adult patients. Clin Chim Acta 2008;387:128-32. [PubMed]
- Briasoulis A, Bakris GL. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep 2013;15:340. [PubMed]
- Motoyama S, Kondo T, Anno H, et al. Atherosclerotic plaque characterization by 0.5-mm-slice multislice computed tomographic imaging. Circ J 2007;71:363-6. [PubMed]
- Nikolaou K, Alkadhi H, Bamberg F, et al. MRI and CT in the diagnosis of coronary artery disease: indications and applications. Insights Imaging 2011;2:9-24. [PubMed]
- Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006;52:5-18. [PubMed]
- K/DOQI Clinical Practice Guidelines on Chronic Kidney Disease. Work Group and Evidence Review Team Membership. American Journal of Kidney Diseases 2002;39:S1-266. [PubMed]
- Matt D, Scheffel H, Leschka S, et al. Dual-source CT coronary angiography: image quality, mean heart rate, and heart rate variability. AJR Am J Roentgenol 2007;189:567-73. [PubMed]
- Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5-40. [PubMed]
- Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32. [PubMed]
- Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol 2005;16:529-38. [PubMed]
- Parfrey PS, Foley RN, Harnett JD, et al. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 1996;11:1277-85. [PubMed]
- Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int 2000;58:353-62. [PubMed]
- Muntner P, Hamm LL, Kusek JW, et al. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 2004;140:9-17. [PubMed]
- Rivera JJ, Nasir K, Cox PR, et al. Association of traditional cardiovascular risk factors with coronary plaque sub-types assessed by 64-slice computed tomography angiography in a large cohort of asymptomatic subjects. Atherosclerosis 2009;206:451-7. [PubMed]
- Kawai H, Sarai M, Motoyama S, et al. Coronary plaque characteristics in patients with mild chronic kidney disease. Analysis by 320-row area detector computed tomography. Circ J 2012;76:1436-41. [PubMed]
- Liu H, Yan L, Ma GS, et al. Association of chronic kidney disease and coronary artery disease in 1,010 consecutive patients undergoing coronary angiography. J Nephrol 2012;25:219-24. [PubMed]
- Cho I, Min HS, Chun EJ, et al. Coronary atherosclerosis detected by coronary CT angiography in asymptomatic subjects with early chronic kidney disease. Atherosclerosis 2010;208:406-11. [PubMed]
- Mitsutake R, Miura S, Shiga Y, et al. Is chronic kidney disease associated with coronary artery stenosis or calcification as assessed by multi-detector row computed tomography? Intern Med 2008;47:1835-41. [PubMed]
- Pundziute G, Schuijf JD, van Velzen JE, et al. Assessment with multi-slice computed tomography and gray-scale and virtual histology intravascular ultrasound of gender-specific differences in extent and composition of coronary atherosclerotic plaques in relation to age. Am J Cardiol 2010;105:480-6. [PubMed]
- Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998;31:126-33. [PubMed]
- Mautner SL, Mautner GC, Froehlich J, et al. Coronary artery disease: prediction with in vitro electron beam CT. Radiology 1994;192:625-30. [PubMed]
- Yiu KH, de Graaf FR, van Velzen JE, et al. Different value of coronary calcium score to predict obstructive coronary artery disease in patients with and without moderate chronic kidney disease. Neth Heart J 2013;21:347-53. [PubMed]


