Contrast-enhanced ultrasound after endovascular aortic repair—current status and future perspectives
Introduction
An increasing number of patients with abdominal aortic aneurysms (AAAs) are undergoing endovascular aortic repair (EVAR) as opposed to open surgery. EVAR can be associated with both early and late post-operative complications—as such, lifelong imaging surveillance is required. In particular, the development of endoleaks can pose a significant threat to patient morbidity and mortality.
Endoleaks are defined as persistent flow of blood around the stent graft leading to aneurysm sac filling. Five different types of endoleaks are distinguished. Type I is related to a leak at the attachment site of the graft resulting in pressure increase within the aneurysm sac. This results in high risk for rupture and demands urgent treatment. Type II endoleaks are relatively frequent, accounting for 40% of all cases and are caused by branch arteries of the aorta or iliac artery filling the aneurysm sac (1). For type II endoleaks, observation is the management of choice with regular follow-up as half of these endoleaks will thrombose spontaneously without need for further intervention (2). During observation, if the size of the aneurysm sac in type II endoleak increases, treatment should be pursued (2). Type III endoleaks result from endograft failure leading to pressure increase in the aneurysm sac, similar to type I endoleaks. Type III endoleaks also necessitate active treatment. Type IV endoleaks are restricted to the intraoperative or early postoperative phase (defined as within 30 days) and do not play a role in the long-term follow-up. Type V endoleaks are idiopathic in nature and are described by high pressure within the aneurysm sac without any appreciable cause. Management of type V endoleaks remains complex and not standardized at this time.
In summary, treatment is required in type I and III endoleaks as these are at high risk for rupture; type II endoleaks are considered to be lower risk and can be observed until the aneurysm sac diameter begins to increase. For leaks requiring intervention, treatment usually involves an endovascular approach. Importantly, the configuration of the aorta of these patients needs to be assessed not only post EVAR but also prior to performing the procedure as some patients are not candidates for EVAR (3,4). The dominant imaging modality in this setting is currently computed tomography angiography (CTA) of the abdomen with IV contrast material. Given potential CT contrast agent side effects and cumulative radiation exposure, an improvement in imaging protocol and strategies is of utmost importance to avoid long-term sequelae.
Contrast-enhanced ultrasound (CEUS)
CEUS is an evolving imaging modality that is gaining popularity in different settings of vascular medicine (3,5). Particularly, CEUS allows for superior visualization of the aorta and its main branches. More importantly, due to the previously mentioned complications of CT, CEUS is a highly attractive tool for the evaluation of patients who have previously undergone EVAR. The endograft can be depicted with CEUS over approximately 5-10 minutes from different angles to assess the perfused lumen and also evaluate low flow states with a high temporal resolution. Thrombotic material can be appreciated as a focal filling defect, located at the wall of the AAA or adjacent to the endograft.
The contrast agents used in CEUS are stabilized microspheres consisting of sulfur hexafluoride or perfluorcarbon encapsulated by a phospholipid shell. These microbubbles show non-linear behavior when examined on an ultrasound machine with low mechanical index (6,7). They serve as true intravascular tracers without extravascular distribution and therefore exhibit blood pool characteristics. These blood pool characteristics can be further enhanced when performing intravenous continuous administration instead of bolus injection (8). Thus, CEUS not only demonstrates detailed morphology post EVAR, but also contributes information with regard to perfusion of the excluded aneurysm sac which aids in detection of post-procedural complications. The microspheres contrast agents are then excreted via the respiratory tract and do not affect renal function, which is of high clinical relevance in the post-EVAR population. At this time, however, sulfur hexafluoride or perfluorocarbon based contrast agents are not FDA approved for this indication. Finally, CEUS does not subject patients to radiation.
While considering these benefits, however, it is important to note that CEUS shares limitations of all ultrasound study modalities. Importantly, CEUS is operator dependent and image quality can be impaired by patient habitus and significant amounts of overlying bowel gas. In addition, CEUS is largely unavailable in the United States with the exception of select academic centers involved in ongoing research. CEUS has been performed internationally for evaluation and surveillance of EVAR patients and data is being collected in the context of a global population (9,10).
Surveillance imaging after EVAR
Surveillance imaging is required post-EVAR due to long-term complications, in particular endoleaks which can lead to stent graft migration and increased secondary aneurysm rupture risk. Endoleaks affect up to 50% of the post-EVAR population, and can happen at any time, making lifelong imaging surveillance necessary (11-14). Therefore, in this section we will discuss the timing and frequencies of different surveillance imaging methods including the corresponding toxicities and costs.
The current imaging standard is repetitive CTA evaluation at 30 days, 6 months and yearly thereafter (14). CTA with an arterial phase and delayed phase is highly sensitive and specific for the detection of complications post EVAR, but this modality cannot appreciate blood flow directions limiting specificity for classification (15). In 2011, the society of vascular surgery (SVS) released clinical practice guidelines regarding surveillance imaging for patients undergoing EVAR (16). Importantly, many of these guidelines were based on consensus of opinions which varied widely. In the absence of any abnormalities on 30-day post-operative imaging, recommended surveillance time was anywhere from every one to five years. Presumably, opinions skewed toward longer surveillance intervals were at least partly based on concerns for cumulative radiation and iodinated nephrotoxic contrast exposure associated with conventional CTA imaging.
Further, long-term effects of CTA need to be considered. Patients receive pre- and post-operative CTAs. Repetitive scans are associated with a significant amount of cumulative radiation exposure and also with the administration of multiple doses of nephrotoxic contrast agents (17). It is well known that in atherosclerotic patients the preservation of the kidney function has a major impact on overall prognosis, and therefore limiting patient exposure to nephrotoxins is crucial (3).
Color Doppler ultrasound has been suggested as an alternative to CTA for EVAR surveillance as it is able to depict in-stent flow (18). The sensitivity of this modality ranges from around 40% to 97% (19-21). Meta-analyses demonstrated a sensitivity of 69% and 77%, respectively using color Doppler ultrasound for the detection of endoleaks (22,23). These data suggest that color Doppler ultrasound alone has insufficient diagnostic capabilities for endoleak detection.
A recently published study, introduced as a standard institutional protocol for EVAR follow-up, demonstrated that duplex ultrasound and radiographs of the abdomen (plain films) can be helpful for graft migration and limb kinking. If an abnormality is detected, or if the duplex ultrasound is non-diagnostic, then CTA can be performed. The authors appreciated discordant or unresolved findings in 33 out of 539 patients post EVAR (approximately 6%) after imaging with duplex ultrasound and CTA, and suggested CEUS for final evaluation. This study showed that CEUS could resolve the clinical questions in all cases. In fact, 10 of these 33 (30%) required a secondary intervention based on the information derived from the CEUS study. The value of CEUS was in this study, related to clarification of endoleak detection and target vessel patency (24).
Costs associated with imaging studies play an increasing role in the modern healthcare system. Gray and colleagues assessed the costs and performance of color duplex ultrasound vs. CTA regarding the detection of endoleaks and residual aneurysm sac size. Color duplex ultrasound post EVAR led to a significant reduction in costs without compromise in diagnostic accuracy (25). Namely, they found that Color duplex ultrasound which is much cheaper than CTA revealed a sensitivity of 100% and a specificity of 85%, a positive predictive value of 28% and a negative predictive value of 100% for endoleak detection vs. CTA. A high correlation was appreciated between both techniques for residual aneurysm sac size measurements.
Furthermore, MR angiography (MRA) has also been suggested as an alternative imaging modality for postoperative surveillance after EVAR. This imaging modality also allows assessing similar to CTA luminal patency, stent positioning, endoleaks, and residual aneurysma size (26). A systemic review revealed that MRA may be more sensitive compared to CTA for the detection of post-EVAR endoleaks, especially for the detection of type II endoleaks (27). Particularly blood-pool MRI contrast agents have prolonged intravascular retention and may be helpful to detect endoleaks with very slow flow and of small volume (28). Therefore, MRA are mostly recommended in cases of endotension where no endoleak has been documented on CTA despite aneurysm sac growth after EVAR. In these cases MRA may detect type II endoleaks. Similar to ultrasound, MRA has the advantage in long-term EVAR surveillance of the lack of ionizing radiation compared to CTA. However, in the clinical setting, MRA is limited by imaging artifacts from ferromagnetic stents, limited availability, higher costs, and longer scan time.
Endoleak detection with CEUS
Besides the mentioned standard ultrasound including B-mode and color Doppler ultrasound the additional use of ultrasound contrast agent has gained great importance in the detection of endoleaks in the last years.
Several studies and meta-analyses have focused on the role of CEUS in endoleak detection. Early studies and case series have revealed promising potential of CEUS for the detection of endoleaks. In one study, CEUS not only confirmed the presence of 20 endoleaks previously discovered by CTA, but also detected two more endoleaks, missed by CTA. The presence of these two endoleaks was confirmed by conventional angiography as gold standard (29). Another study demonstrated the value of CEUS for visualization of low flow endoleaks in patients with increasing aneurysm sac diameter. The authors took advantage of delayed echo enhancement (more than 150 seconds post ultrasound contrast agent administration) to depict the endoleaks, and their presence was again confirmed with digital subtraction angiography as gold standard (30). These findings were concordant with our experience that CEUS is able to detect even subtle endoleaks, including slow flow and small ones if delayed imaging is included in the protocol with microbubbles as contrast agents which have blood pool characteristics. This is a very important advantage of CEUS compared to standard ultrasound and even CTA.
In different studies CEUS compared to CTA has very high sensitivity and specificity for the detection of endoleaks. In a large study of over 100 patients undergoing surveillance imaging with CEUS and other imaging modalities versus conventional angiography as a reference standard for the detection of endoleaks, diagnostic accuracy of CEUS was superior to color Doppler ultrasound and equal to CTA and MRA (31). A smaller prospective study enrolled 35 patients with longitudinal follow-up comparing CTA and CEUS at 1 month and 6 months post-EVAR and yearly thereafter. However, CTA was used as a reference standard in this study as opposed to standard angiography. In regards to endoleak detection by CEUS, sensitivity was 97%, specificity was 100%, positive predictive value was 100%, negative predictive value was 98% and accuracy was 99%. Correct classification by CEUS was reached in 26 out of 33 (79%) endoleaks; no clinically relevant endoleak was missed. The authors concluded that post-EVAR CEUS can be used as follow-up imaging modality in patients with stable or decreasing aneurysm sac size without evidence for endoleak at CTA. This conclusion was based on the high sensitivity, specificity and accuracy demonstrated by the study results. However, the authors also emphasized the role of CTA after CEUS to confirm endoleak classification before a patient undergoes invasive conventional angiography (10). Another prospective study evaluated endoleak detection and classification, demonstrating for CEUS a sensitivity of 92% and a specificity of 100%. CEUS also assessed the endograft with regard to patency and diameter properly, demonstrating a sensitivity of 72% and specificity of 100%. Clinical decisions based on CEUS as opposed to CTA were not different. Therefore the author recommended integrating CEUS in post EVAR surveillance (32).
CEUS in comparison to CTA was also assessed for follow-up of procedures involving fenestrated EVAR. Good agreement with regard to aneurysm sac diameter and complete agreement for target vessel evaluation between both modalities were appreciated. Endoleaks were detected in 7 out of 62 (11%) cases and in 6 out of 62 (10%) cases with CTA and CEUS, respectively. For post fenestrated EVAR surveillance it has been shown that CEUS is as accurate as CTA for the assessment of aneurysm sac diameter and target vessels, as well as detection of endoleaks based on these study results (33). A large retrospective study consisting of 171 patients compared multi-slice CTA versus CEUS post EVAR for the detection and classification of endoleaks revealed a sensitivity of 97% and a specificity of 93%. Thus, CEUS performed comparable to multi-slice CTA in this post EVAR study (34).
Three-dimensional CEUS is currently being introduced to the clinical arena. The technique includes the collection of ultrasound reflections taking advantage of positional information from magnetic field emitters and resulting eventually in a three-dimensional image. Three-dimensional CEUS was compared to CTA for post EVAR imaging, demonstrating a sensitivity of 100%, a specificity of 92%, a positive predictive value of 94% and a negative predictive value of 100% for endoleak detection. A high correlation for AAA sac diameter between CTA and three-dimensional CEUS was found (9). If three dimensional CEUS has any advantage compared with two dimensional CEUS imaging in the setting of EVAR surveillance has to be demonstrated in future studies.
The comparison of normal CEUS imaging with standard ultrasound and CT has already been summarized in a different meta-analysis. A pooled sensitivity of 98% and a pooled specificity of 88% was found for CEUS endoleak detection concluding that CEUS is superior to standard B-mode ultrasound for this indication (22). Another meta-analysis incorporated 11 studies calculating a pooled sensitivity of 96% and a pooled specificity of 85% for CEUS vs. CT for endoleak evaluation. A pooled sensitivity of 74% and a pooled specificity of 94% were found when comparing color Doppler ultrasound vs. CT. When focusing on the clinically important type I and III endoleaks, the pooled sensitivity was 99% and the pooled specificity was 100% for CEUS. For color Doppler ultrasound the pooled sensitivity was 83% and the pooled specificity was 100% when focusing on the clinically important type I and III endoleaks. CEUS was more sensitive but less specific than color Doppler ultrasound (35). Further color Doppler ultrasound delivers less morphologic information when compared to CEUS. CEUS appears more helpful for type II endoleak assessment in comparison to color Doppler ultrasound (35,36).
An overview of a selection of cohort studies and meta-analysis evaluating the sensitivity and specificity of CEUS to detect endoleaks are given in the Table 1.
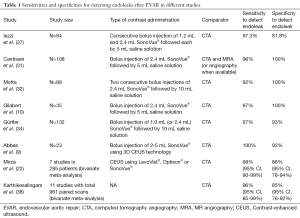
Full table
In summary CEUS revealed at least equal performance compared to CTA for endoleak detection and classification. Some studies show some benefits of CEUS over CTA, in particular for subtle and slow flow endoleaks.
Case studies
Technique and institutional CEUS protocol
CEUS as follow-up was performed in all cases with repetitive bolus of maximum 2.4 mL of SonoVue (Bracco Altana Pharma, Milan, Italy) via intravenous injection. Depending on the sensitivity of the equipment and the constitution of the patient, the dose can be decreased to 1.0 mL or increased up to 4.8 mL. Intraluminal arterial enhancement usually becomes detectable between 10 to 30 seconds after bolus injection and peaks at approximately 30 to 90 seconds, depending on the individual intra-abdominal presentability, and gradually decreases to background within the next 5 minutes. Bolus injection can be repeated every 3-5 minutes. Alternatively, in order to achieve a stable enhancement over several minutes, a continuous infusion (VueJect®, Bracco Milan) of 1-2 mL per minute may be applied after initial bolus injection. The ultrasound equipment for aortic applications with ultrasound contrast agent usually contains a low frequency curved array (e.g., 1 to 5 MHz) in conjunction with a low-mechanical-index contrast-specific ultrasound mode (e.g., pulse inversion harmonic imaging, power modulation) which is mandatory for CEUS. These non-linear pulsing schemes are designed specifically for suppressing the echoes from the tissue while detecting only bubble echoes (3).
Case #1: detection of type II endoleak by CTA and continuation of follow-up with CEUS
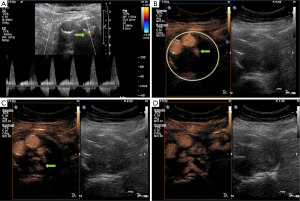
A 72-year-old gentleman with a past medical history of ischemic stroke, coronary artery disease status post drug eluting stent was diagnosed with an infrarenal aneurysm by abdominal B-mode ultrasound. CTA at the time of diagnosis showed an infrarenal aortic aneurysm measuring 6.1 cm at maximum diameter. EVAR was performed with an Endurant stent-graft (Medtronic Cardiovascular, Santa Rosa, California, USA) placed suprarenally proximal and fixed bi-iliacally distal. Three months post procedure, CTA follow-up detected a type II endoleak just below and dorsal to the right prosthetic limb, presumably of a lumbar artery or the inferior mesenteric artery with a maximum extension of the aneurysm sack of 6.2 cm. The patient was asymptomatic and decision for observational approach was made. Three CTAs were performed post diagnosis with type II endoleak at 8, 19, 33 months. Each CTA failed to show any increase in aneurysm sac size and the patient remained asymptomatic. Per patient request, follow-up was switched to CEUS only which was performed at 33 months post-diagnosis with type II endoleak detected. At this assessment, CEUS not only showed the type II endoleak but also revealed a lumbar artery and not the inferior mesenteric artery as origin of the endoleak (Figure 1). The maximum extension of the aneurysm sac was stable on 5.8 cm. This case shows the value of CEUS not only as accurate imaging modality for follow-up after EVAR but also for identifying the source of the type II endoleak whereas the CT could not appreciate the source clearly (lumbar artery or inferior mesenteric artery).
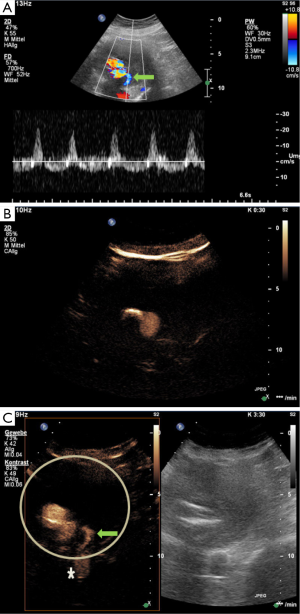
Case #2: detection of type II endoleak by CTA, confirmed at conventional angiography and follow-up with CEUS
A 76-year-old gentleman with a past medical history of seropositive late-onset rheumatoid arthritis on methotrexate and steroids was admitted for cardiac decompensation in the setting of severe aortic stenosis. To rule out aortic dissection CT imaging was performed. The CT revealed incidentally a dilatation of the ascending aorta, with a maximum diameter of 4.5 cm, and of the abdominal aorta with a maximum diameter of 8.5 cm. One day later, open surgery was performed with biological aortic valve replacement and replacement of the ascending aorta. Two weeks post-operatively, EVAR was perforemd using a Gore Excluder device (WL Gore & Associates Inc, Sunnyvale, California, USA) with suprarenal and bi-iliac fixation with elongation of both prosthetic limbs due to complicated arterial vessel configuration leading to implantation of additional four stent grafts. CTA at 1 day and at 4 months post-procedure were unremarkable without evidence of an endoleak. Eleven months after the initial procedure, the patient was still asymptomatic but follow-up CTA revealed an increase in aneurysm sac diameter and suspected type II endoleak of a lumbar artery. Confirmation of the suspected endoleak was obtained by an elective conventional angiography conducted 13 months after endograft implantation. Due to complex vessel structure, the aneurysm sac was not accessible for embolization. As such, a Gore Excluder limb device (WL Gore & Associates Inc, Sunnyvale, California, USA) placed by retrograde access through the left common femoral artery was performed to elongate the left prosthetic limb. CEUS (protocol described in case #1) 1 day post-procedure revealed an unchanged type II endoleak just below the prosthetic body, which was still fed by a lumbar artery. The maximum diameter of the aneurysm sac remained stable at a diameter of 8.7 cm. The study is shown in Figure 2. The patient is scheduled for an observational approach with a short term follow-up in 3 months, with standard ultrasound and CEUS. This case shows that CEUS is useful also for complex anatomy after EVAR and is comparable with the gold standard (angiography) for the detection of endoleaks.
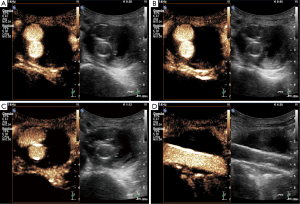
Case #3: CEUS for follow-up imaging studies after EVAR in a patient with severe co-morbidities including chronic kidney failure
An 83-year-old gentleman with a past medical history of a severe coronary artery disease and multiple myocardial infarctions complicated by development of severely impaired systolic left ventricular function, atrial fibrillation, chronic kidney disease status post unilateral nephrectomy 30 years ago was found to have an asymptomatic infrarenal AAA detected during routine diagnostic procedures. Imaging evaluation including CTA revealed a maximum diameter of the aneurysm sac of 6.5 cm. EVAR was performed using a Gore Excluder device (WL Gore & Associates Inc, Sunnyvale, California, USA) with infrarenal and bi-iliac fixation. Initial post-interventional CTA showed a functionally adequate endoprothesis without endoleak. Three months after implantation, the patient remained asymptomatic and CTA showed a slight decrease in the size of the aneurysm sac. Due to progressive chronic kidney disease follow-up was switched to annual conventional ultrasound including CEUS, which demonstrated a well-functioning stent graft without evidence of an endoleak (Figure 3). The patient continues to tolerate yearly CEUS studies well and is currently asymptomatic.
Conclusions: do we need a stronger role of CEUS in EVAR surveillance?
CTA is not an ideal lifelong surveillance diagnostic tool due to the cumulative radiation exposure and the nephrotoxicity and allergic side effects associated with iodine contrast (39-41). CEUS as an evolving modality in this setting is highly attractive and ready to be used based on data from several studies and meta-analyses. CEUS enables real-time evaluation of blood flow with reasonable high spatial resolution for abdominal aortic imaging. According to our experience, as well as reviewed literature, CEUS after EVAR seems to be a good alternative imaging modality to CTA and should be a first-line diagnostic tool for follow-up. However, CEUS cannot completely replace CTA for endoleak assessment. If abnormalities such as endoleak development or an increase in aneurysm sac size are appreciated with CEUS, CTA should be performed followed by angiography for therapeutic purposes. Further, it needs to be considered that CEUS alone in the post-EVAR surveillance may be insufficient and a combination diagnostic algorithm can be implemented.
Therefore, a revised post-EVAR surveillance imaging protocol based on the aforementioned studies should be considered. Based on the very high sensitivity and specificity of CEUS to detect endoleaks comparable to CTA, one plausible strategy would be to perform CEUS imaging post-EVAR as first line surveillance imaging modality combined with periodical routine CTA studies. Once an abnormality on CEUS is detected, CTA should be performed for further work-up and planning of the therapy (42).
Acknowledgments
Funding: Dr. Staub is supported by grants from the Swiss National Science Foundation (Grant PZ00P3_142419 and PBZHB-120997), the Swiss Society of Angiology, the University of Basel.
Footnote
Conflicts of Interest: Dr. Staub is supported by an unrestricted research grant from Bracco Suisse SA, Manno, Switzerland. The other authors have no conflicts of interest to declare.
References
- Cao P, De Rango P, Verzini F, et al. Endoleak after endovascular aortic repair: classification, diagnosis and management following endovascular thoracic and abdominal aortic repair. J Cardiovasc Surg (Torino) 2010;51:53-69. [PubMed]
- Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 2011;41 Suppl 1:S1-S58. [PubMed]
- Staub D, Partovi S, Imfeld S, et al. Novel applications of contrast-enhanced ultrasound imaging in vascular medicine. Vasa 2013;42:17-31. [PubMed]
- Santosa F, Moysidis T, Nowak T, et al. Endovascular abdominal aneurysm repair: trends in Germany. Vasa 2012;41:268-74. [PubMed]
- Aschwanden M, Partovi S, Jacobi B, et al. Assessing the end-organ in peripheral arterial occlusive disease-from contrast-enhanced ultrasound to blood-oxygen-level-dependent MR imaging. Cardiovasc Diagn Ther 2014;4:165-72. [PubMed]
- Staub D, Partovi S, Schinkel AF, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology 2011;258:618-26. [PubMed]
- Partovi S, Loebe M, Aschwanden M, et al. Contrast-enhanced ultrasound for assessing carotid atherosclerotic plaque lesions. AJR Am J Roentgenol 2012;198:W13-9. [PubMed]
- Henao EA, Hodge MD, Felkai DD, et al. Contrast-enhanced Duplex surveillance after endovascular abdominal aortic aneurysm repair: improved efficacy using a continuous infusion technique. J Vasc Surg 2006;43:259-64; discussion 264. [PubMed]
- Abbas A, Hansrani V, Sedgwick N, et al. 3D contrast enhanced ultrasound for detecting endoleak following endovascular aneurysm repair (EVAR). Eur J Vasc Endovasc Surg 2014;47:487-92. [PubMed]
- Gilabert R, Buñesch L, Real MI, et al. Evaluation of abdominal aortic aneurysm after endovascular repair: prospective validation of contrast-enhanced US with a second-generation US contrast agent. Radiology 2012;264:269-77. [PubMed]
- Rand T, Uberoi R, Cil B, et al. Quality improvement guidelines for imaging detection and treatment of endoleaks following endovascular aneurysm repair (EVAR). Cardiovasc Intervent Radiol 2013;36:35-45. [PubMed]
- Buth J, van Marrewijk CJ, Harris PL, et al. Outcome of endovascular abdominal aortic aneurysm repair in patients with conditions considered unfit for an open procedure: a report on the EUROSTAR experience. J Vasc Surg 2002;35:211-21. [PubMed]
- Buth J, Laheij RJ. Early complications and endoleaks after endovascular abdominal aortic aneurysm repair: report of a multicenter study. J Vasc Surg 2000;31:134-46. [PubMed]
- Shah A, Stavropoulos SW. Imaging Surveillance following Endovascular Aneurysm Repair. Semin Intervent Radiol 2009;26:10-6. [PubMed]
- Fan CM, Rafferty EA, Geller SC, et al. Endovascular stent-graft in abdominal aortic aneurysms: the relationship between patent vessels that arise from the aneurysmal sac and early endoleak. Radiology 2001;218:176-82. [PubMed]
- Lee WA, Matsumura JS, Mitchell RS, et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg 2011;53:187-92. [PubMed]
- Verhoeven EL, Oikonomou K, Ventin FC, et al. Is it time to eliminate CT after EVAR as routine follow-up? J Cardiovasc Surg (Torino) 2011;52:193-8. [PubMed]
- Blom AS, Troutman D, Beeman B, et al. Duplex ultrasound imaging to detect limb stenosis or kinking of endovascular device. J Vasc Surg 2012;55:1577-80. [PubMed]
- Sato DT, Goff CD, Gregory RT, et al. Endoleak after aortic stent graft repair: diagnosis by color duplex ultrasound scan versus computed tomography scan. J Vasc Surg 1998;28:657-63. [PubMed]
- Raman KG, Missig-Carroll N, Richardson T, et al. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg 2003;38:645-51. [PubMed]
- d'Audiffret A, Desgranges P, Kobeiter DH, et al. Follow-up evaluation of endoluminally treated abdominal aortic aneurysms with duplex ultrasonography: validation with computed tomography. J Vasc Surg 2001;33:42-50. [PubMed]
- Mirza TA, Karthikesalingam A, Jackson D, et al. Duplex ultrasound and contrast-enhanced ultrasound versus computed tomography for the detection of endoleak after EVAR: systematic review and bivariate meta-analysis. Eur J Vasc Endovasc Surg 2010;39:418-28. [PubMed]
- Ashoke R, Brown LC, Rodway A, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther 2005;12:297-305. [PubMed]
- Millen A, Canavati R, Harrison G, et al. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg 2013;58:18-23. [PubMed]
- Gray C, Goodman P, Herron CC, et al. Use of colour duplex ultrasound as a first line surveillance tool following EVAR is associated with a reduction in cost without compromising accuracy. Eur J Vasc Endovasc Surg 2012;44:145-50. [PubMed]
- Picel AC, Kansal N. Essentials of endovascular abdominal aortic aneurysm repair imaging: postprocedure surveillance and complications. AJR Am J Roentgenol 2014;203:W358-72. [PubMed]
- Habets J, Zandvoort HJ, Reitsma JB, et al. Magnetic resonance imaging is more sensitive than computed tomography angiography for the detection of endoleaks after endovascular abdominal aortic aneurysm repair: a systematic review. Eur J Vasc Endovasc Surg 2013;45:340-50. [PubMed]
- Von Tengg-Kobligk H, Correa Londono M, Von Allmen R, et al. State-of-the-art of imaging detecting endoleaks post-EVAR with special focus on low-flow endoleaks. J Cardiovasc Surg (Torino) 2014;55:563-79. [PubMed]
- Bendick PJ, Bove PG, Long GW, et al. Efficacy of ultrasound scan contrast agents in the noninvasive follow-up of aortic stent grafts. J Vasc Surg 2003;37:381-5. [PubMed]
- Napoli V, Bargellini I, Sardella SG, et al. Abdominal aortic aneurysm: contrast-enhanced US for missed endoleaks after endoluminal repair. Radiology 2004;233:217-25. [PubMed]
- Cantisani V, Ricci P, Grazhdani H, et al. Prospective comparative analysis of colour-Doppler ultrasound, contrast-enhanced ultrasound, computed tomography and magnetic resonance in detecting endoleak after endovascular abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 2011;41:186-92. [PubMed]
- Motta R, Rubaltelli L, Vezzaro R, et al. Role of multidetector CT angiography and contrast-enhanced ultrasound in redefining follow-up protocols after endovascular abdominal aortic aneurysm repair. Radiol Med 2012;117:1079-92. [PubMed]
- Perini P, Sediri I, Midulla M, et al. Contrast-enhanced ultrasound vs. CT angiography in fenestrated EVAR surveillance: a single-center comparison. J Endovasc Ther 2012;19:648-55. [PubMed]
- Gürtler VM, Sommer WH, Meimarakis G, et al. A comparison between contrast-enhanced ultrasound imaging and multislice computed tomography in detecting and classifying endoleaks in the follow-up after endovascular aneurysm repair. J Vasc Surg 2013;58:340-5. [PubMed]
- Karthikesalingam A, Bahia SS, Patel SR, et al. A systematic review and meta-analysis indicates underreporting of renal dysfunction following endovascular aneurysm repair. Kidney Int 2015;87:442-51. [PubMed]
- Giannoni MF, Fanelli F, Citone M, et al. Contrast ultrasound imaging: the best method to detect type II endoleak during endovascular aneurysm repair follow-up. Interact Cardiovasc Thorac Surg 2007;6:359-62. [PubMed]
- Iezzi R, Basilico R, Giancristofaro D, et al. Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2009;49:552-60. [PubMed]
- Karthikesalingam A, Al-Jundi W, Jackson D, et al. Systematic review and meta-analysis of duplex ultrasonography, contrast-enhanced ultrasonography or computed tomography for surveillance after endovascular aneurysm repair. Br J Surg 2012;99:1514-23. [PubMed]
- Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med 2007;357:2277-84. [PubMed]
- de Jong PA, Mayo JR, Golmohammadi K, et al. Estimation of cancer mortality associated with repetitive computed tomography scanning. Am J Respir Crit Care Med 2006;173:199-203. [PubMed]
- Rengier F, Geisbüsch P, Vosshenrich R, et al. State-of-the-art aortic imaging: part I - fundamentals and perspectives of CT and MRI. Vasa 2013;42:395-412. [PubMed]
- Sternbergh WC 3rd, Greenberg RK, Chuter TA, et al. Redefining postoperative surveillance after endovascular aneurysm repair: recommendations based on 5-year follow-up in the US Zenith multicenter trial. J Vasc Surg 2008;48:278-84; discussion 284-5. [PubMed]


