Fragmented QRS on surface electrocardiogram is not a reliable predictor of myocardial scar, angiographic coronary disease or long term adverse outcomes
Introduction
Up to a quarter of all myocardial infarctions go unrecognized (1,2). Currently, the identification of Q waves on more than two contiguous leads seems to be the best tool we have on electrocardiography to detect prior myocardial infarctions (3). However, Q waves can disappear over time in 25-60% of patients and autopsy studies have failed to show consistent correlation between transmurality of infarct and Q waves (4,5). Additionally, magnetic resonance imaging (MRI) studies have shown that Q waves have low sensitivity for prediction of overall location of scars (6). Thus, there is an unmet need for better and readily available tools to detect prior myocardial injury in daily practice. Alterations in QRS morphology, rsR and its variants called fragmented QRS (fQRS) have been shown to occur on surface electrocardiogram (EKG) by studies many years ago (7,8). More recently, fQRS was demonstrated to have high sensitivity and specificity to detect myocardial scar on single-photon emission computed tomography (SPECT) perfusion imaging (4). However, despite recent retrospective studies demonstrating that the presence of fQRS in patients with ischemic cardiomyopathy is a reliable marker of infarct size, the evidence is conflicting regarding the utility of fQRS as one group in a more recent study has refuted an association of fQRS with SPECT scar (2,9). Given this conflicting evidence on fQRS and lack of angiographic correlation to SPECT in prior studies, we aimed to study our patient population undergoing SPECT and coronary angiography (CA) for further defining if fQRS has the capability of predicting SPECT scar and correlate it to CA (10). We further also aimed to study the impact of fQRS on outcomes in these patients over a long term follow-up period of 5 years.
Methods
We retrospectively studied the 12-lead EKGs in 337 consecutive patients undergoing SPECT and CA with known or suspected coronary artery disease (CAD). All EKGs were performed within 1 month of SPECT imaging and all CA studies were performed within 6 months of SPECT. Patients with bundle branch block, paced rhythm or the absence of an EKG within 1 month of the SPECT scan were excluded. All patients had a same day rest-stress Technetium 99m sestamibi or (Myoview, GE Healthcare, Arlington Heights, IL) SPECT with exercise or pharmacologic stress as clinically indicated. SPECT findings were interpreted as normal, abnormal for ischemia (if there were reversible perfusion defects), and scar (if there were fixed defects on rest and stress perfusion imaging associated with wall motion abnormality on gated imaging consistent with scar). All SPECT scans were scored with a 4 point score (0= normal and 4= absent perfusion) using a 17-segment model (11,12).
The EKGs of all patients were analyzed for the presence of fQRS and pathologic Q waves in two or more anatomically contiguous leads by two independent cardiologists blinded to the results of the SPECT scans. The criteria used for fQRS was derived from Das et al. (4) (typically, fQRS is considered to be present when there are changes in QRS morpohology (<120 ms) with varying rsR’ patterns, such as additional R waves, notching of S wave and more than 1 R’ wave. Pathologic Q waves were defined as any Q wave >0.04 seconds and ≥1 mV deep in any two contiguous leads. The coronary distributions assessed included left anterior descending (V1-V5), left circumflex (I, aVL and V6) and right coronary artery (II, III and aVF). The presence of fQRS or Q waves in two contiguous EKG leads was correlated with major coronary artery distributions on SPECT and CA. The data for CA anatomy and lesion severity were retrieved from the official CA report of patients.
A total of 99 patients were excluded due to the presence of a bundle branch block, paced rhythm or the absence of an EKG within 1 month of the SPECT scan. The EKG data (presence of fQRS and Q waves) for the remaining 238 patients were analyzed and compared with SPECT scan to correlation for scar and further angiographic correlation for the presence of significant (>50%) coronary stenosis on CA. Univariable group comparisons (fQRS versus non-fQRS) were made using the chi-square test for non-sparse categorical data, the Fisher exact test for sparse categorical data, 2-sample t-tests for normally distributed numeric data, and Wilcoxon rank sum tests for non-normally distributed numeric data. Variables with group comparison P values less than 0.20 were placed into a full multivariable logistic regression model to predict the presence of fQRS. Cox regression analysis was used to evaluate various study variables as predictors of all-cause mortality and MACE (all-cause death, MI, heart failure, or revascularization) during follow-up. Variables with univariable Cox regression P values less than 0.20 were allowed to participate in a full multivariable Cox regression model. Kaplan-Meier curves for freedom from all-cause mortality and MACE were obtained. Statistical significance was set at P<0.05 throughout. This study protocol was approved by the Institutional Review Board at Henry Ford Health System.
Results
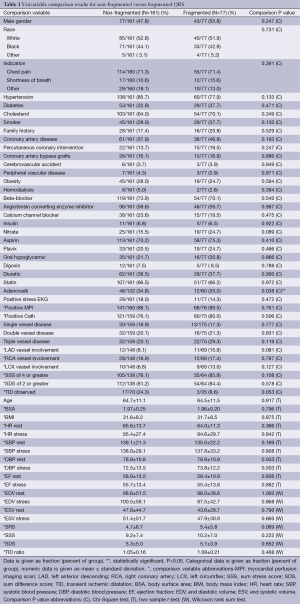
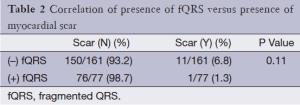
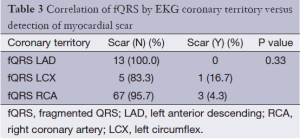
Of the 238 patients’ data analyzed, 77 had fQRS and 161 did not. No differences existed in patient baseline demographic data, medical history, or medication usage between both groups (Table 1). There was no significant difference in the presence of scar on SPECT in the group with (3/77; 3.8%) or without fQRS (11/161; 6.7%) (P=0.56; Table 2). fQRS by EKG coronary distribution was not able to localize scar well with any specific vascular territory by SPECT (Table 3). CA based significant CAD was no different between the fQRS (55/77; 71%) and no fQRS groups (99/161; 61.4%) (P=0.20; Table 4). Similarly, the presence of EKG Q waves was no different in both groups (12/77; 15.5% fQRS and 17/161; 10.5% no fQRS; P=0.3; Table 5).
Full table
Full table
Full table

Full table

Full table
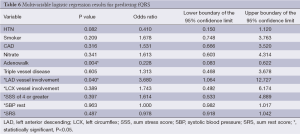
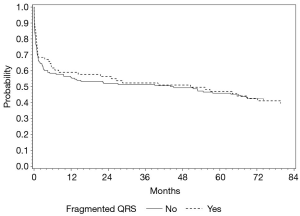
When multivariable analysis was done for predictors of fQRS, it should be noted that in patients with CA based significant left anterior descending (LAD) disease there was a significantly higher likelihood [odds ratio (OR) 3.68] of having the presence of fQRS (P=0.04) on these individual’s baseline EKG (Table 6). However, despite correlation with LAD disease on CA, overall presence of fQRS was not significantly associated with MACE (P=0.92) or specifically all-cause mortality on survival analysis (P=0.93; Figure 1). The absolute number or patients with MACE during follow-up totaled 137 persons, which is 57.6% of the total sample of 238 study patients.
Full table
Discussion
Our study did not demonstrate a definite relationship of fQRS to SPECT scar and significant coronary stenosis (>50%) on CA. Based on our data, we are unable to confirm that fQRS on a 12-lead EKG is a good diagnostic marker of a prior myocardial infarction by SPECT. A prior recent study by our group also has failed to find correlation between fQRS and SPECT scar. The present study extends our previous findings by looking at angiographic disease correlation with fQRS and outcomes, again failing to show a consistent relationship between fQRS, CAD and outcomes (2).
A lot of interest has been shed on fQRS and its correlation to myocardial scar, cardiomyopathy and outcomes over the past few years following the initial study by Das et al. (4) They studied myocardial SPECT for detection of scar in the left ventricle and compared detection of fQRS and Q wave on surface to perfusion defects on SPECT in patients with known CAD and demonstrated that fQRS had higher sensitivity than Q wave but lower specificity to detect SPECT scar (4). In their study, the combination of fQRS and Q wave improved detection of scarred myocardium (4). Other studies have highlighted the role fQRS as a marker of poor collateral development in patients with occluded coronaries, adverse prognosis in CAD patients with wide QRS and in ischemic and non-ischemic cardiomyopathy (9,10,13-16). Furthermore, some have reported that fQRS is a marker of left ventricular aneurysm (17). Numerous other studies have linked fQRS to underlying structural heart diseases and to adverse prognosis (18-21).
However, quite a few studies have reported on the lack of predictive value of fQRS similar to our findings (2,21). A recent study focused on the importance of fQRS in prediction of events in the acute setting following myocardial infarction (22). Investigators in this study evaluated 307 patients with acute myocardial infarction and studied the value of fQRS in predicting MACE. By multivariate analysis, fQRS was not a predictor of MACE, and in univariate analysis; fQRS was not predictive of ventricular arrythmias or heart failure. Our group previously studied fQRS in 460 patients with known or suspected CAD to correlate it to myocardial scar. Similar to prior studies, this was a single center investigation. We adopted the same criteria set forth for fQRS and Q wave morphology as Das et al. (4), but were unable to show correlation of fQRS and SPECT scar. There was overall poor sensitivity, specificity, and positive and negative predictive value to scar and to coronary territory in that study.
One could argue that given the limitations of spatial resolution with SPECT, techniques such as delayed hyperenhancement MRI (DE-MRI) may have better correlation of scar with fQRS. However, this is not the case. Ahn et al. studied 190 patients who underwent DE-MRI and correlated the presence of Q waves and fQRS to the extent of delayed enhancement, and found that despite its inherent high spatial resolution for detecting smaller scars, DE-MRI did not have better correlation for scar with fQRS (23). Delayed enhancement was observed in 180 (94.7%), transmural enhancement was noted in 78 (43.3%) and subendocardial enhancement in 102 (56.7%) patients. The sensitivity and specificity of Q waves and fQRS for diagnosing delayed enhancement were 59.4% vs. 66.7% and 90.0% vs. 40.0%. The receiver operating characteristic curve of delayed enhancement was 0.75 for Q waves and 0.53 for fQRS (P=0.04). The areas under the receiver operating characteristic curves of the transmurality of delayed enhancement were 0.44 for fQRS and 0.58 for Q waves (P=0.73) (23). They concluded that both Q waves and fQRS are poor indicators for extent of transmural myocardial injury/scar (23). In another study from the same group, 86 patients with non-ischemic cardiomyopathy were studied with DE-MRI, and again fQRS showed no relationship with DE MRI in this group of patients (24).
In our current study, apart from comparing SPECT findings with fQRS on EKG, we have further correlated these findings to angiographic anatomy not done in many prior studies. Thus, not only have we shown that fQRS is not a marker for myocardial infarction, as defined by significant Q waves on EKG, and does not correlate to SPECT scar similar to previously mentioned studies, but it does not also correlate with significant CAD on CA and does not predict MACE. Multivariate analysis in our study does indicate that presence of LAD territory involvement as a predictor of the presence of fQRS. The reasons for such discrepancy between our studies are unclear, but likely reflect different definitions of fQRS (exclusion of wide QRS in our study and inclusion in others) and differing patient populations.
Our study was retrospective and hence has its inherent limitations. We used the original definition of fQRS and did not include bundle branch block. Thus, our findings cannot be generalized to patient populations with wide QRS where other authors have reported a modified fQRS criteria. We did not evaluate the value of fQRS in patients with ischemia but mainly focused on scar in our study. As fQRS has been mainly propagated as a marker of structural heart disease, its correlation with structural abnormalities, such as scar, is more relevant rather than transient ischemia. Regardless, by analyzing for CAD on CA greater than 50%, we were able to evaluate all potentially significant lesions and their correlation to fQRS.
Conclusions
Our study does not support the role of fQRS in predicting scar on SPECT or angiographic significant CAD in any specific distribution. Furthermore, fQRS does not appear to reliably predict MACE. Although larger prospective studies may be required to clarify these conflicting findings, it appears that clinicians do not need to work up fQRS on EKG as a separate clinical entity, but rather continue to rely on their clinical assessment and already validated modalities for patient risk assessment in CAD. This study does not support routine assessment of fQRS on surface EKG as a reliable predictor of SPECT myocardial scar, MACE or all-cause mortality over a long period of follow-up.
Acknowledgements
The authors wish to thank Stephanie Stebens for her assistance in manuscript formatting and editing.
Disclosure: The authors declare no conflict of interest.
References
- Yan AT, Tan M, Fitchett D, et al. One-year outcome of patients after acute coronary syndromes (from the Canadian Acute Coronary Syndromes Registry). Am J Cardiol 2004;94:25-9. [PubMed]
- Wang DD, Buerkel DM, Corbett JR, et al. Fragmented QRS complex has poor sensitivity in detecting myocardial scar. Ann Noninvasive Electrocardiol 2010;15:308-14. [PubMed]
- Sochman J. The electrocardiogram in acute myocardial infarction with reperfusion: current concepts regarding Q waves and their dynamics. Vnitr Lek 2006;52:1181-4. [PubMed]
- Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495-501. [PubMed]
- Yasuda M, Iida H, Itagane H, et al. Significance of Q wave disappearance in the chronic phase following transmural acute myocardial infarction. Jpn Circ J 1990;54:1517-24. [PubMed]
- Rovai D, Di Bella G, Rossi G, et al. Q-wave prediction of myocardial infarct location, size and transmural extent at magnetic resonance imaging. Coron Artery Dis 2007;18:381-9. [PubMed]
- Flowers NC, Horan LG, Tolleson WJ, et al. Localization of the site of myocardial scarring in man by high-frequency components. Circulation. 1969;40:927-34. [PubMed]
- el-Sherif N. The rsR’ pattern in left surface leads in ventricular aneurysm. Br Heart J 1970;32:440-8. [PubMed]
- Lorgis L, Cochet A, Chevallier O, et al. Relationship Between Fragmented QRS and No-Reflow, Infarct Size, and Peri-Infarct Zone Assessed Using Cardiac Magnetic Resonance in Patients With Myocardial Infarction. Can J Cardiol 2014;30:204-10. [PubMed]
- Ozdemir S, Tan YZ, Colkesen Y, et al. Comparison of fragmented QRS and myocardial perfusion-gated SPECT findings. Nucl Med Commun 2013;34:1107-15. [PubMed]
- Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42. [PubMed]
- Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998;97:535-43. [PubMed]
- Kadı H, Ceyhan K, Koç F, et al. Relation between fragmented QRS and collateral circulation in patients with chronic total occlusion without prior myocardial infarction. Anadolu Kardiyol Derg 2011;11:300-4. [PubMed]
- Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol 2008;1:258-68. [PubMed]
- Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm 2010;7:74-80. [PubMed]
- Guo R, Zhang J, Li Y, et al. Prognostic significance of fragmented QRS in patients with non-ST elevation myocardial infarction: results of a 1-year, single-center follow-up. Herz 2012;37:789-95. [PubMed]
- Reddy CV, Cheriparambill K, Saul B, et al. Fragmented left sided QRS in absence of bundle branch block: Sign of left ventricular aneurysm. Ann Noninvasive Electrocardiol. Ann Noninvasive Electrocardiol 2006;11:132-8. [PubMed]
- Femenía F, Arce M, Van Grieken J, et al. Fragmented QRS as a predictor of arrhythmic events in patients with hypertrophic obstructive cardiomyopathy. J Interv Card Electrophysiol 2013;38:159-65. [PubMed]
- Ağaç MT, Korkmaz L, Bektas H, et al. Increased frequency of fragmented QRS in patients with severe aortic valve stenosis. Med Princ Pract 2014;23:66-9. [PubMed]
- Park SJ, Chung S, On YK, et al. Fragmented QRS complex in adult patients with Ebstein anomaly and its association with arrhythmic risk and the severity of the anomaly. Circ Arrhythm Electrophysiol 2013;6:1148-55. [PubMed]
- Kadi H, Kevser A, Ozturk A, et al. Fragmented QRS complexes are associated with increased left ventricular mass in patients with essential hypertension. Ann Noninvasive Electrocardiol 2013;18:547-54. [PubMed]
- Lorgis L, Jourda F, Hachet O, et al. Prognostic value of fragmented QRS on a 12-lead ECG in patients with acute myocardial infarction. Heart Lung 2013;42:326-31. [PubMed]
- Ahn MS, Kim JB, Yoo BS, et al. Fragmented QRS complexes are not hallmarks of myocardial injury as detected by cardiac magnetic resonance imaging in patients with acute myocardial infarction. Int J Cardiol 2013;168:2008-13. [PubMed]
- Ahn MS, Kim JB, Joung B, et al. Prognostic implications of fragmented QRS and its relationship with delayed contrast-enhanced cardiovascular magnetic resonance imaging in patients with non-ischemic dilated cardiomyopathy. Int J Cardiol 2013;167:1417-22. [PubMed]


