
Doppler echocardiography for surveillance of acute cardiac allograft rejection: a 28-year single-center experience
Highlight box
Key findings
• Doppler echocardiography for surveillance of acute cardiac rejection (ACR) does not impair long-term results after heart transplantation.
What is known and what is new?
• Endomyocardial biopsies (EMB) are recommended for the detection of ACR.
• We observed that ultrasound variables correlated with EMB findings for the diagnosis of ACR.
What is the implication, and what should change now?
• A first-line non-invasive approach using echocardiography is a reasonable alternative to systematic EMB for surveillance of heart transplant recipients.
Introduction
Background
Detection of early or subclinical acute cardiac rejection (ACR) remains a major concern in heart transplantation (HT). ACR accounts for nearly 10% of all the fatalities during the first year after transplant and 12% of HT recipients experience at least one treated ACR during the same period (1). Endomyocardial biopsies (EMB) are recommended as the gold standard method for ACR surveillance despite a limited sensitivity due to the patchy histological distribution of rejection (2,3). This invasive procedure is generally repeated more than 12 times during the first year after transplant and is associated with a 6% rate of serious iatrogenic complications, including the risks of tamponade, ventricular arrhythmia, right bundle branch block, air embolism and tricuspid valve injury (2,3). The impact on patient’s quality of life is also questionable, especially in the pediatric population (4).
Rationale and knowledge gap
Noninvasive alternatives to EMB have been proposed to detect ACR. Significant changes in T2 signaling have been associated with rejection on cardiac magnetic resonance imaging. This approach is however limited by its availability and by myocardial water content during the first postoperative weeks after transplant (5,6). Peripheral blood genomic expression profiling has been recently applied for detection of moderate to severe ACR (7,8). Similarly, favorable preliminary results considering cell-free DNA, microRNA and extracellular vesicles have been reported (9-12). These circulating biomarkers share a high negative predictive value, but without specific threshold scores for the diagnosis of ACR to date. Another limitation of molecular monitoring is the delay to get results that may not be compatible with clinicians needs.
Objective
Diagnostic performance of Doppler echocardiography for ACR detection and correlations with histological pattern have been investigated since the late 1980’s. Impairment of left ventricular (LV) diastolic function appears sooner than changes in systolic function in case of ACR (13-16). In 1990, echocardiographic screening of diastolic function was initiated in our institution (Marie Lannelongue Hospital, University of Paris Saclay, France) to monitor cardiac allografts along with conventional EMB within 24 hours. Since 1998, EMB were not routinely performed for surveillance of ACR, while Doppler echocardiography was used as the first-line approach. We herein report the long-term results of this non-invasive diagnostic method, challenging the conventional surveillance for ACR after HT. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-305/rc).
Methods
Study design
We retrospectively enrolled all patients older than 18 years who underwent a HT between January 1990 and December 2018 in our institution (Marie Lannelongue Hospital, Groupe Hospitalier Paris Saint-Joseph). Two consecutive cohorts were defined. The “Dual Monitoring Cohort” (DMC) comprised patients transplanted from January 1990 to December 1997 who underwent routine EMB and Doppler echocardiography within 24 hours for ACR surveillance. From January 1998 to December 2018, Doppler echocardiography was performed, as first-line approach for ACR detection defining the “Echo-First Cohort” (EFC) (Figure 1).
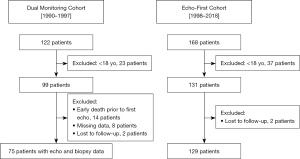
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol complied with the International Society for Heart and Lung Transplantation (ISHLT) Ethics Statement and was approved by the Institutional Ethics Committee of Marie Lannelongue Hospital, Groupe Hospitalier Paris Saint-Joseph (initial agreement No. 28). The study protocol was registered on the National Institute of Health data platform INDS (No. TPS 15256bis) with approval of the national evaluation committee CEREES (French ad hoc evaluation committee). According to the French regulation (JORF No. 0160 of 13 July 2018 text No. 110, MR-004) an information note setting out the purpose of the research has been transmitted to patients. The requirement for informed consent was waived because of the retrospective nature of the study. Patients’ non-opposition to the use of their data for research purposes was collected by mailing in accordance with the European General Data Protection Regulation (GDPR).
Histological standard for rejection status
Tissue samples were obtained from transfemoral EMB. For EMB performed between 1990 and 2005, histology-based ACR was graded using the ISHLT 1990 statement as follows: grades 0 (no rejection), 1A (mild focal), 1B (mild diffuse), 2 (moderate focal), 3A (moderate focal), 3B (moderate diffuse), or 4 (severe) (17). After 2005, we used the revised ISHLT 2005 classification as follows: grades 0 (no rejection), 1 (mild), 2 (moderate) and 3 (severe) (18). Histological diagnosis was done by an experienced pathologist, blindly from echocardiographic findings. Treatment of ACR was considered for grade ≥1B for EMB performed from 1990 to 2005, and for grade ≥1 for EMB performed after 2005.
Echocardiography protocol
Two-dimensional and M-mode echocardiography (Vivid ultrasound system, GE Healthcare, Horten, Norway) combined with pulse wave Doppler analysis for mitral flow were performed by experimented cardiologists, blinded to EMB interpretation. Transplant cardiologists graduated in cardiac echography imaging after completion of a post-doctoral fellowship in cardiology and transplantation medicine. The following measurements of interest for surveillance of ACR were collected: early diastolic (E) wave peak velocity; pressure half time (PHT) as time required for the pressure gradient to decrease to half of its maximal value, which is related to E wave deceleration time (DT); isovolumetric relaxation time (IVRT) defined as the interval between aortic valve closure and mitral valve opening. Consistent with the preliminary results reported by Desruennes et al., evidence for ACR was considered in case of at least one of the following:
- LV diastolic dysfunction defined by IVRT <80 ms, peak E wave >100 cm/s; PHT <50 ms. Significant decrease in IVRT and PHT, and/or significant increase in E wave peak velocity, between two consecutive examinations (≥20% changes) in a same patient were also considered as a marker of impaired diastolic function compatible with early rejection;
- LV systolic dysfunction;
- Acute LV hypertrophy with wall thickness >12 mm combined with sparkling texture of the myocardium (13).
Echography inter-operator variability assessment
Two experienced cardiologists graduated in cardiac ultrasound imaging, but not involved in the follow-up of HT patients, independently reviewed digital video loops and images randomly selected from a panel of twenty HT recipients from the EFC. These operators were blinded to each other. Inter-operator variability of ultrasound measurements for IVRT, PHT and E wave was assessed.
Immunosuppressive regiment and post-transplant follow-up
The immunosuppressive therapy protocol is detailed in Appendix 1. In the DMC, EMB and echocardiography were performed within 24 hours as follows: weekly for the first 5 weeks after transplant, then every 2 weeks for the next 2 months, every 3 weeks for the next 2 months, and then once a month until the end of the first year. Echocardiographic findings were therefore recorded before the announcement of EMB result. In the EFC, Doppler echocardiography was weekly performed during the first 2 months after transplant, then every 2 weeks for 1 month and finally monthly until the end of the first year. Routine echocardiographic screening was thereafter performed every 6 weeks during the second year after transplant, and then every 3 months. EMB were considered in symptomatic patients with normal echocardiography, or in case of moderate changes in LV filling profile with increased plasma level of brain natriuretic peptides since 2005. Coronary angiography was first performed 1 year after transplant and was thereafter repeated every year or when clinically warranted. Since 2005, computed tomography (CT) coronary angiography has been applied for routine surveillance of coronary allograft vasculopathy (CAV) instead of coronary angiography (19). Each time CAV was suspected on noninvasive CT imaging, a coronary angiography was performed. Coronary lesions were scored according to the ISHLT grading score for CAV (20).
Data collection and statistical analysis
Patients’ characteristics were retrospectively reviewed from hospital records. Data were collected from January 2019 to December 2020. Donors’ characteristics were collected from the French national HT database (Agence de la Biomedecine, La Plaine Saint Denis, France). Continuous variables were presented as mean ± standard deviation and differences as mean [95% confidence interval (95% CI)] when normally distributed, and as median [interquartile range (IQR)] when non-normally distributed. Categorical variables were displayed as numbers (%). Unpaired t-test and Wilcoxon test of variance were used to compare continuous variables, while categorical variables were compared using the chi-square or the Fisher test. Survival rates were estimated using the Kaplan-Meier method, and compared with the log-rank test. The follow-up was completed in December 2020. The kappa coefficient was used to test agreement between echography measurements and EMB results (Table S1). Each ultrasound/biopsy pair was defined as an event and numbered chronologically. Inter-operator variability for ultrasound measurements was assessed using a Wilcoxon test and the Kappa coefficient. A P value <0.05 was considered as significant. Receiver operating characteristic (ROC) curves were constructed using the easyROC software. We calculated area under the curve (AUC) (95% CI) according to this program (21). All statistical analyses were performed using the R software v 4.0.5 (www.r-project.org/).
Results
Patient population
A total of 228 patients were included in the study. Pretransplant characteristics of the recipients were not different between the two cohorts, excepted for the etiology of heart failure leading to HT, with a higher rate of valvular heart disease and retransplantation in the recent era (P<0.01) (Table 1). Donor age and body mass index (BMI) were significantly higher in the EFC compared to the DMC (P<0.01 and P=0.013 respectively). There was a trend for a higher rate of trauma, stroke and hypoxia among the causes of donor death in the recent cohort (Table 1). The mean allograft ischemic time was longer in the recent cohort (P<0.001), whereas no difference was remarkable considering gender and cytomegalovirus mismatches.
Table 1
| Variables | Total (n=228) | Cohort 1990–1997 (n=99) | Cohort 1998–2018 (n=129) | Difference (95% CI) | P value* |
|---|---|---|---|---|---|
| Characteristics of the recipients | |||||
| Age (years) | 46.5±13.9 | 48.5±12.8 | 44.9±14.5 | 3.66 (0.03; 7.29) | 0.08 |
| Weight (kg) | 68.9±12.9 | 68.2±11.6 | 69.5±13.9 | −1.29 (−4.69; 2.12) | 0.46 |
| Height (cm) | 171.3±7.9 | 171.0±7.6 | 171.4±8.1 | −0.39 (−2.46; 1.68) | 0.78 |
| BMI (kg/m2) | 23.5±4.1 | 23.2±3.5 | 23.6±4.6 | −0.36 (−1.41; 0.69) | 0.75 |
| Gender | 0.21 | ||||
| Female | 48 (21.1) | 17 (17.2) | 31 (24.0) | −6.86 (−17.33; 3.61) | |
| Male | 180 (78.9) | 82 (82.8) | 98 (76.0) | ||
| Etiology (n=227) | <0.01 | ||||
| Valvular | 50 (22.0) | 17 (17.2) | 33 (25.8) | −8.61 (−20.12; 2.90) | |
| Ischemic | 81 (35.7) | 35 (35.4) | 46 (35.9) | −0.58 (−13.73; 12.56) | |
| Congenital | 19 (8.4) | 14 (14.1) | 5 (3.9) | 10.24 (1.70; 18.77) | |
| Dilated cardiomyopathy | 65 (28.6) | 33 (33.3) | 32 (25.0) | 8.33 (−4.50; 21.17) | |
| Retransplantation | 8 (3.5) | 0 | 8 (6.3) | −6.25 (−11.34; −1.16) | |
| Others | 4 (1.8) | 0 | 4 (3.1) | −3.12 (−7.03; 0.78) | |
| List waiting time (months) | 63.0 [15.8; 166.8] | 70.0 [25.5; 157.0] | 56.0 [8.00; 169.0] | 0.45 | |
| Characteristics of the donors | |||||
| Age (years) | 41.8±13.4 | 38.2±11.7 | 44.6±13.9 | −6.39 (−9.81; −2.97) | <0.01 |
| Weight (kg) (n=225) | 75.5±13.6 | 73.3±12.7 | 77.1±14.0 | −3.77 (−7.34; −0.19) | 0.040 |
| Height (cm) (n=215) | 174.0±7.9 | 174.3±7.3 | 173.7±8.3 | 0.57 (−1.59; 2.73) | 0.72 |
| BMI (kg/m2) (n=215) | 25.0±4.3 | 24.0±3.5 | 25.6±4.6 | −1.55 (−2.64; −0.46) | 0.013 |
| Sex (n=213) | 0.034 | ||||
| Female | 52 (24.4) | 14 (16.7) | 38 (29.5) | −12.79 (−23.99; −1.59) | |
| Male | 161 (75.6) | 70 (83.3) | 91 (70.5) | ||
| Cause of death (n=226) | 0.046 | ||||
| Trauma | 102 (45.1) | 39 (40.2) | 63 (48.8) | −8.63 (−22.56; 5.30) | |
| Suicide | 41 (18.1) | 25 (25.8) | 16 (12.4) | 13.37 (2.07; 24.67) | |
| Stroke | 77 (34.1) | 32 (33.0) | 45 (34.9) | −1.89 (−15.25; 11.47) | |
| Hypoxia | 6 (2.7) | 1 (1.0) | 5 (3.9) | −2.85 (−7.64; 1.95) | |
| Ischemic time (min) (n=226) | 203±51 | 175±45 | 226±59 | −50.09 (−60.40; −39.78) | <0.001 |
| Gender mismatch† (n=213) | 57 (26.8) | 20 (23.8) | 37 (28.7) | 4.87 (−7.12; 16.87) | 0.43 |
| CMV mismatch‡ (n=224) | 108 (48.2) | 40 (42.1) | 68 (52.7) | 10.61 (−2.54; 23.75) | 0.12 |
Data are expressed as mean ± standard deviation, n (%) or median [interquartile range]. †, female donor to male recipients; ‡, donor CMV+ to recipient CMV−; *, unpaired t-test and Wilcoxon test of variance were used to compare continuous variables, while categorical variables were compared using the chi-square or the Fisher test. CI, confidence interval; BMI, body mass index; CMV, cytomegalovirus.
Survival analysis
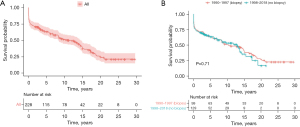
The median follow-up periods were 116 months (IQR, 0–361 months) and 42 months (IQR, 0–261 months) for DMC and EFC respectively. The overall 1-, 5-, 10-, 15- and 20-year survival rates were respectively 74.1%, 65.4%, 55.5%, 44.1% and 32.9% in the study population. There was no significant difference in long-term survival between the two cohorts (log rank test, P=0.71). The comparative survival estimates at 1, 5, 10 and 15 years were 75.0%, 63.0%, 49.0% and 38.0% in the DMC, and 74.0%, 63.0%, 53.0% and 35.0% in the EFC (Figure 2).
Immune-related post-transplant outcomes and incidence of CAV in the EFC
The median delay between transplantation and first episode of acute rejection was 284 days (IQR, 61–914 days) in the EFC. Twenty-three point six percent of patients experienced at least one treated ACR during the first year after transplant and 36.8% during the first 5 years of follow-up (Table S2). During the first year of follow-up, CAV grade 1 was observed in 19.0% of patients in the EFC. CAV grade 1 or more was observed in 52.0% and 81.0% of patients in the EFC at 5 and 10 years respectively. The rate of CAV grade 3 remained lower than 8.0% over the time (Table 2).
Table 2
| CAV grade | 1 year (n=87/94, 93%) | 5 years (n=50/52, 96%) | 10 years (n=27/29, 93%) |
|---|---|---|---|
| CAV 1 | 16 (18%) | 21 (42%) | 13 (48%) |
| CAV 2 | 1 (1%) | 2 (4%) | 7 (26%) |
| CAV 3 | 0 | 3 (6%) | 2 (7%) |
CAV, coronary allograft vasculopathy.
Comparison between histological and ultrasound measurements for rejection surveillance in the DMC
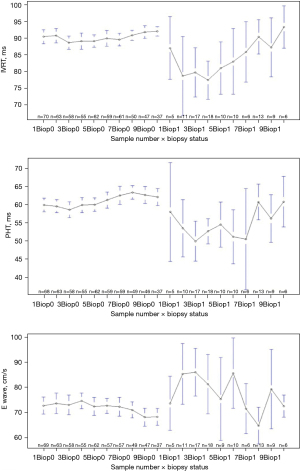
Ultrasound measurements and histological results were considered for DMC patients at each follow-up visit after transplant. The Kappa coefficient was not measurable for the first event and was at 0.56 (IQR, 0.25–0.83, P<0.001), 0.63 (IQR, 0.42–0.85, P<0.001), 0.74 (IQR, 0.55–0.92, P<0.001), 0.53 (IQR, 0.23–0.84, P<0.001), 0.53 (IQR, 0.23–0.83, P<0.001) respectively for the second, third, fourth, fifth and sixth events. Ultrasound measurements were not different whatever the result of the biopsy at the time of the first follow-up examination. IVRT was subsequently decreased when the biopsy was positive at the 2nd and the 3rd examinations (P=0.005 and P=0.05). Similarly, E wave was significantly higher in case of positive biopsy at the 2nd and 3rd examinations (P=0.017 and P=0.010). PHT was significantly lower in case of histologically proven ACR over the same period after transplant (P=0.012 and P=0.002) (Table 3). Temporal diagram showed a stability of the different echocardiographic measurements over the time in patients with negative EMB (Figure 3). In patients with histologically proven ACR, IVRT and PHT were lower compared to patients without ACR, while E wave seemed to be higher, with a wide range of variability. ROC curves for IVRT, PHT and E wave are illustrated in Figure 4 showing significant relationships between ultrasound parameters and positive EMB at the second and third events after transplant.
Table 3
| Ultrasound variables | Negative EMB (0 or 1A) | Positive EMB (1B or 2A or 2) | P value* |
|---|---|---|---|
| 1st event | (n=70) | (n=5) | |
| IVRT (ms) | 90.4±8.8 | 87.0±7.6 | 0.38 |
| PHT (ms) | 60.0±7.8 | 58.0±11.0 | 0.96 |
| E wave (cm/s) | 72.7±14.2 | 73.6±8.7 | 0.75 |
| 2nd event | (n=64) | (n=11) | |
| IVRT (ms) | 90.7±8.7 | 78.6±17.6 | 0.005 |
| PHT (ms) | 59.6±7.0 | 53.5±11.1 | 0.012 |
| E wave (cm/s) | 73.6±16.2 | 85.3±17.9 | 0.017 |
| 3rd event | (n=58) | (n=17) | |
| IVRT (ms) | 88.6±7.5 | 79.7±14.4 | 0.05 |
| PHT (ms) | 58.6±8.3 | 49.9±10.7 | 0.002 |
| E wave (cm/s) | 73.0±14.9 | 85.9±18.4 | 0.010 |
Data are expressed as mean ± standard deviation. *, paired t-test. EMB, endomyocardial biopsy; IVRT, isovolumetric relaxation time; PHT, pressure half time.

Inter-operator variability for ultrasound measurements in the EFC
Inter-operator variability for diastolic ultrasound parameters was investigated by two non-expert cardiologists. Twenty recipients from the EFC were randomly selected. Agreement assessed by the Kappa coefficient was 0.66 (IQR, 0.31–1.00) (P<0.001). Mean IVRT and E wave values were comparable between the two operators, whereas the mean PHT values were significantly different (P=0.001) (Table 4).
Table 4
| Ultrasound variables | Observer A | Observer B | Inter-observer difference (95% CI) | P value* |
|---|---|---|---|---|
| IVRT (ms) | 99.4±16.8 | 102.9±17.9 | −3.5 (−8.44; 1.44) | 0.30 |
| E wave (cm/s) | 85.0±26.0 | 82.0±26.0 | 0.03 (−0.007; 0.06) | 0.13 |
| PHT (ms) | 49.4±13.1 | 43.6±10.9 | 5.8 (2.9; 8.7) | 0.001 |
Data are expressed as mean ± standard deviation. *, paired t-test. CI, confidence interval; IVRT, isovolumetric relaxation time; PHT, pressure half time.
Discussion
Key findings
The main finding of the present study is that long-term survival was not different in patients followed with Doppler echocardiography as a first-line approach for diagnosis of ACR compared to patients monitored with routine EMB. Decision not to systematically perform EMB after HT was not associated with adverse long-term outcomes in our experience. To the best of our knowledge, we report the longest follow-up of HT recipients without systematic EMB.
Strengths and limitations
Our transplant care protocol follows the guidelines of the ISHLT except for surveillance of ACR since we do not perform systematic EMB (22). Our long-term survival estimates are however consistent with the ISHLT registry showing more than 50% survival at 10 years and around 45% at 15 years in the most recent era (23). The present monocentric study includes two successive cohorts of patients over three consecutive decades. Therefore, we cannot exclude an unmeasured bias in both characteristics and medical management of patients. However, the two cohorts are reasonably comparable, and patients did not seem to be over-treated since the incidence of at least one treated episode is consistent with the data from the 2015 ISHLT report (24). The lack of antibody-mediated rejection (AMR) data is an important limitation of this study, mainly related to the absence of AMR diagnosis over the majority of the study period. Treatment of symptomatic AMR is currently recommended, whereas the optimal therapeutic option for asymptomatic AMR remains a matter of debate (25). Our approach has never been validated to date in the setting of AMR, but we hypothesize that AMR may impair LV diastolic function and could therefore be diagnosed at the early stage using ultrasound measurements. Indeed, significant changes in diastolic parameters have been associated with low grade of ACR including intensive vascular rejection phenotype that could be linked to AMR (26). Last, no single parameter seems to be sufficiently sensitive to detect all episodes of acute rejection (27). However, we report a significant correlation between the presence of abnormal ultrasound measurements (PHT, IVRT, E wave) and a positive EMB as illustrated by ROC curves. The lack of sensitivity for ultrasound parameters at the first post-transplant echocardiography could be explained by marked myocardial edema in the early post-operative course.
Comparison with similar researches
Mena et al. underline in a recent meta-analysis the heterogeneity and the small size of cohorts focusing on the benefits of ultrasound measurements for ACR surveillance (28). Diastolic parameters as non-invasive markers of ACR have been introduced during the 1990’s (13-16,29,30). However divergent results have not allowed their application for surveillance of ACR instead of routine EMB. The main concern is the various sensitivity of E wave and PHT, in part explainable by the high prevalence of relaxation disturbances along with progressive decrease in myocardial compliance after HT (e.g., high stiffness due to increased fibrosis due to CAV or microcirculatory dysfunction). This limitation can be addressed by analyzing the variations of diastolic parameters over the time for each patient instead of considering each variable independently.
Our results were favorable despite the higher incidence of CAV in our population (81.0% with CAV grade 1 or more at 10 years) compared to previous studies reporting 40 to 46% of CAV at 10 years (25,31). These findings are consistent with those recently reported by Loupy et al. underscoring that older organ donors in Europe share a higher risk of transmitted coronary artery disease for the recipient (32).
Explanations of findings
In our experience, PHT and IVRT were markedly decreased in patients with positive EMB. These results are consistent with the preliminary studies from Desruennes et al. demonstrating a specificity and a sensitivity of 87% and 87% respectively for 20% PHT decrease and 85% and 90% for 20% IVRT decrease (13,16). EMB is the gold standard diagnostic approach to which alternatives methods, such as Doppler echocardiography, are compared. However, the limited sensitivity of EBM along with its inter-operator variability has been pointed out several times (33-35). The prevalence of ‘biopsy-negative’ ACR (echocardiographic and hemodynamic features suggestive of significant ACR which become reversible by ACR therapy) can reach 20% (36,37). Current ISHLT guidelines recommend to treat all symptomatic rejections defined by clinical symptoms or graft dysfunction (systolic dysfunction), and to treat asymptomatic rejection only when it is moderate (ACR > grade 2R). This statement is consistent with our non-invasive approach using Doppler echocardiography since we observed significant changes in ultrasound measurements when EMB were positive for ACR ≥ grade 1B. Our findings seem to support that diastolic parameters could be more sensitive to detect subclinical ACR as previously suggested by other groups (14,15). In accordance with Dandel et al., we believe that our approach may not underestimate the incidence of ACR and that our patients may not be undertreated in case of subclinical acute rejection (38).
Implications and actions needed
The risk of iatrogenic complications along with the limited sensitivity of EMB has to be considered when challenging the potential benefit of alternative approaches for ACR surveillance. The incidence of asymptomatic ACR requiring treatment is moreover lower than 2% of the overall performed biopsies as reported by Hamour et al. (2) Considering the low incidence of asymptomatic rejections diagnosed by EMB, the sensibility and specificity of ultrasound diastolic parameters would promote Doppler echocardiography as a relevant alternative approach for ACR surveillance. Its application in clinical practice is however limited by inter-operator variability. In our study, the concordance between non-experienced cardiologists (not trained for our ultrasound protocol in HT recipients) was remarkable. There were significant differences only for PHT measurements, probably because this parameter is the most difficult to assess due to its variability over the cardiac cycle. Our favorable long-term results furthermore underscore the reproducibility of our protocol over the past three decades. The present approach was initially introduced and practiced by a single cardiologist (L.H.), while our HT recipients are currently followed using the same ultrasound protocol by four other cardiologists without impairment of our results. In the light of this long-term clinical experience, we trust that our protocol could be widely applied by other groups. From a general point of view, non-invasive monitoring of ACR could make life-long surveillance more acceptable for HT recipients (39).
Conclusions
In the present study, the use of Doppler echocardiography as a first-line approach for surveillance of ACR did not impair long-term results after HT. These findings suggest that this non-invasive approach might be a reasonable alternative to systematic EMB, limiting risk and improving patient quality of life. These encouraging results warrant prospective validation in larger cohorts to be widely accepted by the community of HT caregivers.
Acknowledgments
We thank the Clinical Research Department on behalf of the Groupe Hospitalier Paris Saint Joseph for its scientific, regulatory and technical support. This manuscript is dedicated to organ donor families and to organ procurement organizations.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-305/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-305/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-305/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-305/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol complied with the International Society for Heart and Lung Transplantation (ISHLT) Ethics Statement, and was approved by the Institutional Ethics Committee of Marie Lannelongue Hospital, Groupe Hospitalier Paris Saint-Joseph (initial agreement No. 28). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for informed consent was waived because of the retrospective nature of the study. Patients’ non-opposition to the use of their data for research purposes was collected by mailing in accordance with the European General Data Protection Regulation (GDPR).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant 2018;37:1155-68. [Crossref] [PubMed]
- Hamour IM, Burke MM, Bell AD, et al. Limited utility of endomyocardial biopsy in the first year after heart transplantation. Transplantation 2008;85:969-74. [Crossref] [PubMed]
- From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc 2011;86:1095-102. [Crossref] [PubMed]
- Godown J, McKane M, Mettler BA, et al. Increased Frequency of Surveillance Biopsy Does Not Improve Pediatric Heart Transplant Outcomes: Time for a Change in Practice? J Heart Lung Transplant 2016;35:S73. [Crossref]
- Taylor AJ, Vaddadi G, Pfluger H, et al. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur J Heart Fail 2010;12:45-51. [Crossref] [PubMed]
- Olymbios M, Kwiecinski J, Berman DS, et al. Imaging in Heart Transplant Patients. JACC Cardiovasc Imaging 2018;11:1514-30. [Crossref] [PubMed]
- Crespo-Leiro MG, Stypmann J, Schulz U, et al. Clinical usefulness of gene-expression profile to rule out acute rejection after heart transplantation: CARGO II. Eur Heart J 2016;37:2591-601. [Crossref] [PubMed]
- Moayedi Y, Foroutan F, Miller RJH, et al. Risk evaluation using gene expression screening to monitor for acute cellular rejection in heart transplant recipients. J Heart Lung Transplant 2019;38:51-8. [Crossref] [PubMed]
- Castellani C, Burrello J, Fedrigo M, et al. Circulating extracellular vesicles as non-invasive biomarker of rejection in heart transplant. J Heart Lung Transplant 2020;39:1136-48. [Crossref] [PubMed]
- Constanso-Conde I, Hermida-Prieto M, Barge-Caballero E, et al. Circulating miR-181a-5p as a new biomarker for acute cellular rejection in heart transplantation. J Heart Lung Transplant 2020;39:1100-8. [Crossref] [PubMed]
- Hidestrand M, Tomita-Mitchell A, Hidestrand PM, et al. Highly sensitive noninvasive cardiac transplant rejection monitoring using targeted quantification of donor-specific cell-free deoxyribonucleic acid. J Am Coll Cardiol 2014;63:1224-6. [Crossref] [PubMed]
- Ragalie WS, Stamm K, Mahnke D, et al. Noninvasive Assay for Donor Fraction of Cell-Free DNA in Pediatric Heart Transplant Recipients. J Am Coll Cardiol 2018;71:2982-3. [Crossref] [PubMed]
- Desruennes M, Corcos T, Lechat P, et al. Evaluation by Doppler echocardiography of left ventricular diastolic function in acute graft rejection after heart transplantation. Arch Mal Coeur Vaiss 1988;81:193-8. [PubMed]
- Dodd DA, Brady LD, Carden KA, et al. Pattern of echocardiographic abnormalities with acute cardiac allograft rejection in adults: correlation with endomyocardial biopsy. J Heart Lung Transplant 1993;12:1009-17. [PubMed]
- Valantine H, Fowler M, Hatle L, et al. Doppler echocardiographic indices of diastolic function as markers of acute cardiac rejection. Transplant Proc 1987;19:2556-9. [PubMed]
- Desruennes M, Solis E, Cabrol A, et al. Doppler echocardiography: an excellent noninvasive method for the detection of acute cardiac allograft rejection. Transplant Proc 1989;21:3634-8. [PubMed]
- Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant 1990;9:587-93. [PubMed]
- Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005;24:1710-20. [Crossref] [PubMed]
- Romeo G, Houyel L, Angel CY, et al. Coronary stenosis detection by 16-slice computed tomography in heart transplant patients: comparison with conventional angiography and impact on clinical management. J Am Coll Cardiol 2005;45:1826-31. [Crossref] [PubMed]
- Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant 2010;29:717-27. [Crossref] [PubMed]
- Goksuluk D, Korkmaz S, Zararsiz G, et al. easyROC: An Interactive Web-tool for ROC Curve Analysis Using R Language Environment. R J 2016;8:213-30. [Crossref]
- Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29:914-56. [Crossref] [PubMed]
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report - 2019; focus theme: Donor and recipient size match. J Heart Lung Transplant 2019;38:1056-66. [Crossref] [PubMed]
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1244-54. [Crossref] [PubMed]
- Kfoury AG, Stehlik J, Renlund DG, et al. Impact of repetitive episodes of antibody-mediated or cellular rejection on cardiovascular mortality in cardiac transplant recipients: defining rejection patterns. J Heart Lung Transplant 2006;25:1277-82. [Crossref] [PubMed]
- Hammond EH, Yowell RL, Nunoda S, et al. Vascular (humoral) rejection in heart transplantation: pathologic observations and clinical implications. J Heart Transplant 1989;8:430-43. [PubMed]
- Lu W, Zheng J, Pan X, et al. Diagnostic performance of echocardiography for the detection of acute cardiac allograft rejection: a systematic review and meta-analysis. PLoS One 2015;10:e0121228. [Crossref] [PubMed]
- Mena C, Wencker D, Krumholz HM, et al. Detection of heart transplant rejection in adults by echocardiographic diastolic indices: a systematic review of the literature. J Am Soc Echocardiogr 2006;19:1295-300. [Crossref] [PubMed]
- Ciliberto GR, Cataldo G, Cipriani M, et al. Echocardiographic assessment of cardiac allograft rejection. Eur Heart J 1989;10:400-8. [Crossref] [PubMed]
- Morocutti G, Di Chiara A, Proclemer A, et al. Signal-averaged electrocardiography and Doppler echocardiographic study in predicting acute rejection in heart transplantation. J Heart Lung Transplant 1995;14:1065-72. [PubMed]
- Sato T, Kato TS, Komamura K, et al. Utility of left ventricular systolic torsion derived from 2-dimensional speckle-tracking echocardiography in monitoring acute cellular rejection in heart transplant recipients. J Heart Lung Transplant 2011;30:536-43. [Crossref] [PubMed]
- Loupy A, Coutance G, Bonnet G, et al. Identification and Characterization of Trajectories of Cardiac Allograft Vasculopathy After Heart Transplantation: A Population-Based Study. Circulation 2020;141:1954-67. [Crossref] [PubMed]
- Nielsen H, Sørensen FB, Nielsen B, et al. Reproducibility of the acute rejection diagnosis in human cardiac allografts. The Stanford Classification and the International Grading System. J Heart Lung Transplant 1993;12:239-43. [PubMed]
- Yang HM, Lai CK, Gjertson DW, et al. Has the 2004 revision of the International Society of Heart and Lung Transplantation grading system improved the reproducibility of the diagnosis and grading of cardiac transplant rejection? Cardiovasc Pathol 2009;18:198-204. [Crossref] [PubMed]
- Marboe CC, Billingham M, Eisen H, et al. Nodular endocardial infiltrates (Quilty lesions) cause significant variability in diagnosis of ISHLT Grade 2 and 3A rejection in cardiac allograft recipients. J Heart Lung Transplant 2005;24:S219-26. [Crossref] [PubMed]
- Miller CA, Fildes JE, Ray SG, et al. Non-invasive approaches for the diagnosis of acute cardiac allograft rejection. Heart 2013;99:445-53. [Crossref] [PubMed]
- Subherwal S, Kobashigawa JA, Cogert G, et al. Incidence of acute cellular rejection and non-cellular rejection in cardiac transplantation. Transplant Proc 2004;36:3171-2. [Crossref] [PubMed]
- Dandel M, Hummel M, Meyer R, et al. Left ventricular dysfunction during cardiac allograft rejection: early diagnosis, relationship to the histological severity grade, and therapeutic implications. Transplant Proc 2002;34:2169-73. [Crossref] [PubMed]
- Danziger-Isakov L, Frazier TW, Worley S, et al. Perceived barriers to medication adherence remain stable following solid organ transplantation. Pediatr Transplant 2019;23:e13361. [Crossref] [PubMed]


