
Right ventricle to pulmonary artery conduit: a comparison of long-term graft-related events between bovine jugular vein conduit, aortic homograft, and porcine-valved conduits
Highlight box
Key findings
• Bovine jugular vein should be avoided for patients at high risk of infective endocarditis or underdeveloped pulmonary artery branches and in these cases; homograft could be the conduit of choice.
What is known, and what is new?
• The smaller right ventricle to pulmonary artery conduits, the more risk for reintervention risk factor.
• In our study cohort, we reported that smaller conduit sizes were associated with an increased risk of operative mortality
What is the implication, and what should change now?
• The association between age and the increased risk of reoperation could suggest using other palliative procedures to postpone conduit implantation until the patients get older to decrease the risk of reoperation and operative mortality.
Introduction
Right ventricle to pulmonary artery (RV-PA) construction is an integral surgical procedure in the biventricular repair of several congenital heart diseases, including tetralogy of Fallot, pulmonary atresia, and truncus arteriosus (1-3). Reinterventions, either surgical or transcatheter, after RV-PA construction is common, and the optimal RV-PA conduit still does not exist (4,5). Several grafts are available for RV-PA reconstruction, including aortic and pulmonary homografts (6). However, the number of organ donors limited the use of homografts on a wide scale. Consequently, new alternatives have been introduced, including bovine jugular vein (BJV) conduits (Contegra, Medtronic, Minneapolis) and the composite porcine valve in the Dacron tube (Hancock, Medtronic, Minneapolis) (7-10).
BJV conduits are available in small sizes, making them suitable for neonates; additionally, they have a lower cost than homografts (11). Furthermore, BJV could have comparable durability to homografts. Herrmann and colleagues reported that BJV conduits had better freedom from reoperation compared to aortic homografts, with no difference between BJV conduits and pulmonary homografts (12). However, the risk of infective endocarditis is of concern with BJV conduits. Beckerman and associates reported an incidence of 10% of endocarditis rate at a median follow-up of 7.5 years with BJV conduits (13).
Several factors affect the longevity and the event-free survival of the RV-PA conduits that may be patients or conduit-related. The optimal conduit is still a matter of continuous debate, and tailoring conduit choice according to the patient’s specific risk factors could improve the outcomes of RV-PA reconstruction.
This study compared RV-PA reconstruction using BJVs, aortic homografts, and porcine-valved conduits. Additionally, we reported different types of graft-related events after RV-PA reconstruction and their potential risk factors. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-364/rc).
Methods
Patients
This retrospective cohort study included 155 patients who had 193 procedures for implanting RV-PA conduits from 1999 to 2021 at King Faisal Specialist Hospital and Research Center, Jeddah, Saudi Arabia. Three types of conduits were used: BJVs Medtronic® (Group 1, n=153), aortic homografts CenoValve®(Group 2, n=29), and porcine-valved conduits Carpentier Edwards® (Group 3, n=11). All patients had both orthotopic and heterotopic conduit implantation and biventricular repair. Patients with RV-PA reconstruction using synthetic conduits and those with the univentricular repair were excluded. The graft choice was dependent on surgeons’ preference and experience and the available graft sizes. Patients who had RV-PA conduit with single ventricle pathway were excluded.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the local ethical committee of King Faisal Specialist Hospital and Research Center-Jeddah (IRB 2022-36). The ethical committee waived the need for informed consent for the retrospective nature of the study.
Data and outcomes
Preoperative data included age in months, gender, weight, diagnosis, and pulmonary blood flow. Baseline data were collected at the time of the indexed procedure. The type and size of the conduits were reported. The study outcomes were hospital mortality and follow-up graft-related events. Graft-related events included infective endocarditis, transcatheter pulmonary valve replacement (PVR), transcatheter conduit dilatation, surgical conduit replacement, and transcatheter pulmonary branch intervention.
Balloon dilation for severe conduit stenosis was indicated if right ventricular (RV) pressure was more than 60% of systemic pressure. Balloon dilation or stenting of peripheral pulmonary branch stenosis was required when lung perfusion showed one lung with less than 25% perfusion compared to the other lung or RV pressure of more than 60% of systemic pressure (14). Transcatheter PVR was indicated if symptomatic pulmonary regurgitation or asymptomatic with magnetic resonance imaging parameters of RV end-diastolic indexed volume >150 mL/m2, Z-score >4, RV end-systolic indexed volume >80 mL/m2, RV ejection fraction (EF) <47%, left ventricular EF <55% or large RV outflow tract aneurysm or ECG criteria of QRS duration >160 ms or sustained tachyarrhythmia. Conduit replacement was performed if the medical treatment of infective endocarditis failed, or cardiac catheter-based interventions were not possible.
Follow-up
The patients were followed in the outpatient clinics and the follow-up data were retrieved from the medical charts. Additionally, follow-up data were collected from admission and procedure records for patients who required readmission of interventions. Patients followed by phone calls to confirm the vital status, as a part of the clinical follow-up.
Statistical analysis
The continuous data were compared between the three groups using the one-way analysis of variance (ANOVA) for variables with equal variance and the Kruskal-Wallis test in case of unequal variance. Bartlett’s test was used to assess the homogeneity of variance. The Bonferroni test was used for posthoc analysis after ANOVA and Dunn’s test after the Kruskal-Wallis test. The adjusted P value cutoff for multiple comparison was 0.0167. Categorical data were compared with the Chi-squared or Fisher exact test. Univariable logistic regression analysis was used to evaluate factors associated with operative mortality, and odds ratios were reported. Time to events data were plotted using the Kaplan-Meier curve and compared with the log-rank test. Factors associated with graft-related events were evaluated using univariable and multivariable stepwise Cox regression analysis. All variables were included in the multivariable regression with a forward selection method, variables with a P value of less than 0.1 were retained in the final model, and hazard ratios were reported. Data were presented as median and (25th and 75th percentiles) or absolute frequency and percentages. Missing data were considered missing completely at random. Stata 17 (Stata Corp, College Station, TX, USA) was used to perform all analyses, and factors of a two-sided P value of less than 0.05 was considered statistically significant.
Results
Baseline and conduit characteristics
Gender was equally distributed among groups, and most patients were males. Patients in Group 1 were significantly younger than Group 3 (P=0.009), with no difference between other groups. The weight of patients in Group 1 was significantly lower than Group 2 (P=0.002) and Group 3 (P<0.001), with no difference in weight between Groups 2 and 3 (P=0.081).
The most frequent diagnosis in Group 1 and Group 2 was pulmonary valve atresia [72 (47.06%) and 17 (58.62%), respectively], and in Group 3 was tetralogy of Fallot (7, 63.64%). There was no difference in the diagnosis of additional lesions among groups. The additional lesions were absent pulmonary valve (n=6), atrioventricular septal defect (n=2), dextrocardia (n=1), Down syndrome (n=1), Fanconi syndrome (n=1), Marfan syndrome (n=2), mesocardia (n=1) and situs inversus totalis (n=2). There was no difference in the pulmonary blood flow among the groups.
The conduit size was significantly smaller in Group 1 than Group 3 (P<0.001) and Group 2 (P<0.001), with no difference between Groups 2 and 3 (P=0.084) (Table 1).
Table 1
| Characteristics | Group 1 (n=153) | Group 2 (n=29) | Group 3 (n=11) | P value |
|---|---|---|---|---|
| Male | 88 (57.52) | 16 (55.17) | 6 (54.55) | 0.96 |
| Age (months) | 21 [8–45]; P(vs. group 2): 0.364 | 40 [12–130]; P(vs. group 3): 0.232 | 90 [18–220]; P(vs. group 1): 0.009 | 0.006 |
| Weight (kg) | 9 [6–15]; P(vs. group 2): 0.002 | 15 [9–25]; P(vs. group 3): 0.081 | 25 [12–60]; P(vs. group 1): <0.001 | <0.001 |
| Diagnosis | 0.06 | |||
| TOF | 31 (20.26) | 3 (10.34) | 7 (63.64) | |
| Pulmonary atresia | 72 (47.06) | 17 (58.62) | 2 (18.18) | |
| TGA, VSD and PS | 17 (11.11) | 2 (6.90) | 1 (9.09) | |
| Truncus arteriosus | 26 (16.99) | 4 (13.79) | 1 (9.09) | |
| Left-side lesion | 7 (4.58) | 3 (10.34) | 0 | |
| Additional lesion | 14 (9.15) | 0 | 2 (18.18) | 0.09 |
| Pulmonary flow | 0.29 | |||
| Normal | 0 | 1 (3.45) | 0 | |
| Overflow | 28 (18.42) | 5 (17.24) | 1 (9.09) | |
| Low flow | 124 (81.58) | 23 (79.31) | 10 (90.91) | |
| Conduit size (mm) | 14 [14–16] (n=150); P(vs. group 2): <0.001 | 19 [15–21] (n=27); P(vs. group 3): 0.084 | 20 [18–23]; P(vs. group 1): <0.001 | <0.001 |
Data were presented as median [25th–75th percentile] or numbers and percentages. Group 1, bovine jugular vein; Group 2, homograft; Group 3, porcine valved conduit. PS, pulmonary stenosis; TOF, tetralogy of Fallot; TGA, transposition of great arteries; VSD, ventricular septal defect.
Operative mortality
Operative mortality occurred in 13 patients: 12 (7.84%) with BJV conduit and 1 (9.09%) with porcine-valved conduit (P=0.351). No mortality was reported in patients with aortic homograft. Univariable analysis revealed that male gender [odds ratio (OR): 10.04; 95% confidence interval (CI): 1.28–78.86; P=0.028] and smaller conduit size (OR: 0.78; 95% CI: 0.61–0.99; P=0.048) were associated with increased operative mortality (Table 2).
Table 2
| Risk factors | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| Male | 10.04 (1.28–78.86) | 0.03 |
| Age | 0.98 (0.95–1.004) | 0.10 |
| Weight | 0.91 (0.82–1.01) | 0.08 |
| Diagnosis | 1.51 (0.96–2.38) | 0.08 |
| Additional lesion | 0.92 (0.11–7.54) | 0.936 |
| Conduit type | 0.61 (0.17–2.26) | 0.47 |
| Conduit size | 0.78 (0.61–0.99) | 0.048 |
| Pulmonary blood flow | 0.52 (0.16–1.68) | 0.27 |
Follow-up
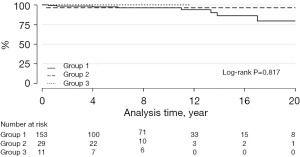
The median follow-up was 84 months (IQR: 33–127 months). Infective endocarditis of the graft occurred in 9 patients: 8 with BJVs and 1 with an aortic homograft (log-rank P=0.817) (Figure 1).
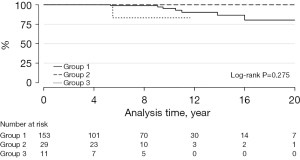
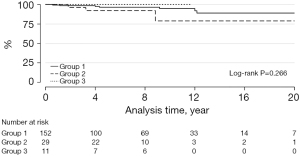
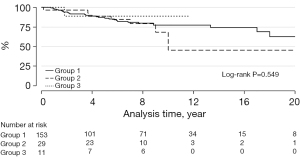
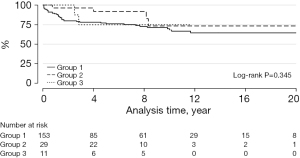
Transcatheter PVR was required in 8 patients: 7 with BJVs conduits and 1 with porcine-valved conduit (P=0.275) (Figure 2). Transcatheter conduit dilatation was performed in 10 patients: 7 with BJVs and 3 with homografts (P=0.266) (Figure 3). Thirty-eight patients had conduit replacement: 29 with BJVs, 8 with homografts, and 1 with porcine-valved conduits (P=0.549). Freedom from conduit replacement at 2, 5, and 10 years was 94%, 86%, and 78% in Group 1, 97%, 85%, and 45% in Group 2, and 89%, 89%, and 89% in Group 3 (Figure 4). Peripheral pulmonary branch interventions were required in 46 patients: 40 with BJVs, 4 with homografts and 2 with porcine-valved conduit (P=0.345). Balloon dilatation of pulmonary branches was performed in 7 patients (17.50%) with BJVs, 2 with homografts (50%), and 2 with porcine-valved conduit (100%). Stenting of the peripheral pulmonary artery branches was performed in 33 patients (82.50%) with BJVs, 2 with aortic homografts (50%) (P=0.012). Freedom from peripheral pulmonary branch interventions at 2, 5, and 10 years was 80%, 67%, and 68% in Group 1, 96%, 92%, and 73% in Group 2, and 100%, 75%, and 75% in Group 3 (Figure 5).
At least one graft-related event was reported in 85 conduits, 69 with BJVs, 12 with homografts, and 4 with porcine-valved conduits (P=0.919). Freedom from graft-related events at 2, 5, and 10 years was 76%, 67%, and 52% in Group 1, 86%, 74%, and 36% in Group 2, and 89%, 53%, and 53% in Group 3 (Figure 6). Factors associated with increased graft-related events were male gender (HR: 1.58; 95% CI: 1.004–2.50; P=0.048) and younger age (OR =0.995; 95% CI: 0.991–0.999; P=0.041) (Table 3).
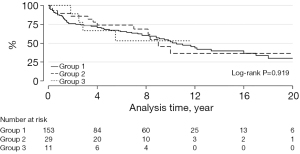
Table 3
| Risk factor | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | P value | Hazard ratio (95% confidence interval) | P value | ||
| Male | 1.48 (0.95–2.29) | 0.080 | 1.58 (1.004–2.50) | 0.048 | |
| Age | 0.99 (0.992–0.999) | 0.036 | 0.995 (0.991–0.999) | 0.04 | |
| Weight | 0.98 (0.97–1.002) | 0.094 | – | – | |
| Diagnosis | 1.06 (0.88–1.28) | 0.540 | – | – | |
| Additional lesion | 0.87 (0.38–1.99) | 0.739 | – | – | |
| Conduit type | 0.93 (0.61–1.39) | 0.715 | – | – | |
| Conduit size | 0.97 (0.90–1.04) | 0.419 | – | – | |
| Restricted pulmonary flow | 1.45 (0.77–2.73) | 0.246 | 1.87 (0.97–3.62) | 0.06 | |
Discussion
The different RV-PA conduits available for RV outflow tract reconstruction fall into one of five categories: (I) homograft conduit (pulmonary and aortic); (II) stented xenograft conduit; (III) stentless xenograft conduit; (IV) autologous tissue conduit; and (V) expanded polytetrafluoroethylene valved conduit. Although different RV-PA conduits are available, the ideal graft has yet to be developed. This study compared the most commonly used conduits for RV-PA reconstruction; BJVs, aortic homografts, and porcine-valved conduits. Individualizing conduit selection based on patient factors may result in improved outcomes.
BJVs were the most common conduits used for young age, which could be attributed to the availability of small sizes compared to other graft types (11). Conduit choice at a young age is affected by several factors, including graft durability and availability of small sizes. Vitanova et al. compared the durability of RV-PA conduits for RV-PA reconstruction in patients below 1 year of age (15). They included 145 patients under one year with BJVs, homografts, or porcine-valved conduits. The freedom from conduit exchange did not differ significantly among groups. BJV conduits developed moderate conduit stenosis or regurgitation faster than other conduit types. They also reported that younger age (<1 month) was a risk factor for conduit replacement (13). Lewis and colleagues reported that smaller conduits size and smaller patients’ age and weight at the time of surgery were risk factors for conduit replacement (16).
Additionally, we reported that smaller conduit sizes were associated with an increased risk of operative mortality. Bonilla-Ramirez reported that smaller RV-PA conduits were a reintervention risk factor (17). The findings about the association between age and the increased risk of reoperation could suggest using other palliative procedures to postpone conduit implantation until the patients get older to decrease the risk of reoperation.
Despite being non-significant, the risk of infective endocarditis was higher after 10 years with BJV conduits specially in patients with bad dental hygiene. Mery et al. studied 586 patients who had 792 conduits, including pulmonary homograft, aortic homograft, BJVs, and porcine-valved conduits (18). They reported a higher infective endocarditis rate in patients with BJVs compared to other conduits. Another study also confirmed this finding; the risk of infective endocarditis was higher with BJVs, Melody valves, and patients with previous RV-PA reconstruction (19). Lewis and colleagues reported that the risk of endocarditis was lower with pulmonary homograft compared to BJV conduits (16). These results suggest that BJVs should be avoided in patients with a high risk of infections, such as those with previously infective endocarditis or DiGeorge syndrome.
Pulmonary valve stenosis or regurgitation is a potential clinical problem after RV-PA reconstruction. The condition can be safely managed with transcatheter PVR (20,21). In our series, we did not report a significant difference among the three conduits in transcatheter PVR; however, the rate seems to increase after 10 years with BJV conduits, and aortic homograft was the least conduit type associated with PVR. Skoglund et al., in a study from the Swedish registry, reported that conduits replacement became the most common intervention performed on RV-PA conduits after the introduction of transcatheter PVR (22).
Interventions on peripheral pulmonary branches were the most common graft-related event in our series. We did not report a difference in peripheral pulmonary branch interventions among groups; however, the Kaplan-Meier curve showed that early interventions were required more frequently in BJV conduits, and aortic homografts had late reinterventions. The endovascular approach became the main management of peripheral pulmonary branch stenosis (23). In our series, stenting was the most common method used, especially in patients with BJV conduits.
Reinterventions are common after RV-PA reconstruction, and intervention-free survival is low (24). The freedom from graft-related events after 10 years in our series was 52% for BJVs, 36% for aortic homografts, and 53% for porcine-valved conduits. A tailored approach for each patient could help to decrease the reintervention rate. Younger age could be a risk factor for reintervention; therefore, delaying conduit insertion by using palliative procedures or patching of the pulmonary artery or RV outflow tract could be considered (25). Endovascular interventions may be used to delay the need for conduit replacement, such as stenting of the pulmonary artery branches and transcatheter conduit dilatation (26-28). BJVs should not be considered for patients at high risk of infective endocarditis, and homograft could be the conduit of choice. From our experience, the risk of endocarditis could be down syndrome or DiGeorge syndrome, history of infective endocarditis, dental caries or abscess and failure to thrive below 3rd percentile for weight.
In summary, the best conduit is what fits well. From our data we found that if there are risk factors for infective endocarditis, it might be better to use homograft. Homograft is also valid if the pulmonary branches are undeveloped or smaller in size. If the patient is young or the plan to do less surgical reoperation, it might be better to use BJV conduit.
Future perspectives
This study found that reintervention is common after RV-PA reconstruction. Endovascular interventions may be used to delay the need for conduit replacement, such as stenting of the pulmonary artery branches and transcatheter conduit dilatation. We reported relatively more infection with BJVs; therefore, they should not be considered for patients at high risk of infective endocarditis, and homografts could be the conduit of choice. This study provided insight into tailored conduit selection, and further studies are required to optimize conduit selection further and assess pulmonary homografts, which was not evaluated in our study because they are not available in our institution.
Study limitations
Several limitations should be considered when interpreting the results of this study. First, the study is retrospective with its inherent biases. However, this is a suitable design to evaluate the long-term outcomes of different RV-PA conduits because of the procedure’s relative rarity. Second, the imbalance of the number of conduits in each group. This imbalance could be attributed to several factors, including the preference for BJVs in young patients due to the availability of small sizes and other conduits in older patients and patients requiring conduit replacement. Third, the study is a single-center experience, and the results could be affected by surgeons’ experience. Lastly, all homografts used in our study were aortic, and we did not use pulmonary homografts, which could be superior to other conduits in this position.
The surgical techniques and equipment advanced with time, as well as the surgical skills. This could be a factor that may affect outcomes. On the other hand, complexity of the cases and the number of patients who required reinterventions also increased with time.
Conclusions
RV-PA reconstruction was associated with low mortality, unrelated to the conduit type. Reinterventions for graft-related events were common. The durability of BJV grafts was an advantage for this type of conduit but on expenses to have more frequent other cardiac catheterization interventions and risk of infective endocarditis. Factors associated with increased graft-related events in this study were male gender and younger age.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-364/rc
Data Sharing Statement: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-364/dss
Peer Review File: Available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-364/prf
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-23-364/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the local ethical committee of King Faisal Specialist Hospital and Research Center-Jeddah (IRB 2022-36). The ethical committee waived the need for informed consent for the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alsoufi B. Right ventricle-to-pulmonary artery conduits: Do we really have an option? J Thorac Cardiovasc Surg 2016;151:442-3. [Crossref] [PubMed]
- Alamri RM, Dohain AM, Arafat AA, et al. Surgical repair for persistent truncus arteriosus in neonates and older children. J Cardiothorac Surg 2020;15:83. [Crossref] [PubMed]
- Elmahrouk AF, Ismail MF, Arafat AA, et al. Outcomes of biventricular repair for shone's complex. J Card Surg 2021;36:12-20. [Crossref] [PubMed]
- Mashali MH, Yousef AA, Elmahrouk AF, et al. Reintervention after repair of tetralogy of Fallot: a one-decade single-center experience. Cardiothorac Surg 2023;31:28. [Crossref]
- Willetts RG, Stickley J, Drury NE, et al. Four right ventricle to pulmonary artery conduit types. J Thorac Cardiovasc Surg 2021;162:1324-1333.e3. [Crossref] [PubMed]
- Saxena A, Salve GG, Betts K, et al. Outcomes Following Heterotopic Placement of Right Ventricle to Pulmonary Artery Conduits. World J Pediatr Congenit Heart Surg 2021;12:220-9. [Crossref] [PubMed]
- Scavo VA Jr, Turrentine MW, Aufiero TX, et al. Valved bovine jugular venous conduits for right ventricular to pulmonary artery reconstruction. ASAIO J 1999;45:482-7. [Crossref] [PubMed]
- Singh A, de Leval M, Stark J. Total correction of type I truncus arteriosus in a 6-month-old infant. Br Heart J 1975;37:1314-6. [Crossref] [PubMed]
- Poinot N, Fils JF, Demanet H, et al. Pulmonary valve replacement after right ventricular outflow tract reconstruction with homograft vs Contegra®: a case control comparison of mortality and morbidity. J Cardiothorac Surg 2018;13:8. [Crossref] [PubMed]
- Ismail MF, Elmahrouk AF, Arafat AA, et al. Bovine jugular vein valved xenograft for extracardiac total cavo-pulmonary connection: The risk of thrombosis and the potential liver protection effect. J Card Surg 2020;35:845-53. [Crossref] [PubMed]
- Brown JW, Ruzmetov M, Rodefeld MD, et al. Valved bovine jugular vein conduits for right ventricular outflow tract reconstruction in children: an attractive alternative to pulmonary homograft. Ann Thorac Surg 2006;82:909-16. [Crossref] [PubMed]
- Herrmann JL, Larson EE, Mastropietro CW, et al. Right Ventricular Outflow Tract Reconstruction in Infant Truncus Arteriosus: A 37-year Experience. Ann Thorac Surg 2020;110:630-7. [Crossref] [PubMed]
- Beckerman Z, De León LE, Zea-Vera R, et al. High incidence of late infective endocarditis in bovine jugular vein valved conduits. J Thorac Cardiovasc Surg 2018;156:728-734.e2. [Crossref] [PubMed]
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021;42:563-645. [Crossref] [PubMed]
- Vitanova K, Cleuziou J, Hörer J, et al. Which type of conduit to choose for right ventricular outflow tract reconstruction in patients below 1 year of age?†. Eur J Cardiothorac Surg 2014;46:961-6; discussion 966. [Crossref] [PubMed]
- Lewis MJ, Malm T, Hallbergson A, et al. Long-Term Follow-Up of Right Ventricle to Pulmonary Artery Biologic Valved Conduits Used in Pediatric Congenital Heart Surgery. Pediatr Cardiol 2023;44:102-15. [Crossref] [PubMed]
- Bonilla-Ramirez C, Ibarra C, Binsalamah ZM, et al. Right Ventricle to Pulmonary Artery Conduit Size Is Associated with Conduit and Pulmonary Artery Reinterventions After Truncus Arteriosus Repair. Semin Thorac Cardiovasc Surg 2022;34:1003-9. [Crossref] [PubMed]
- Mery CM, Guzmán-Pruneda FA, De León LE, et al. Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J Thorac Cardiovasc Surg 2016;151:432-9, 441.e1-2.
- Gröning M, Tahri NB, Søndergaard L, et al. Infective endocarditis in right ventricular outflow tract conduits: a register-based comparison of homografts, Contegra grafts and Melody transcatheter valves. Eur J Cardiothorac Surg 2019;56:87-93. [Crossref] [PubMed]
- Alkashkari W, Albugami S, Abbadi M, et al. Transcatheter pulmonary valve replacement in pediatric patients. Expert Rev Med Devices 2020;17:541-54. [Crossref] [PubMed]
- Wagner R, Daehnert I, Lurz P. Percutaneous pulmonary and tricuspid valve implantations: An update. World J Cardiol 2015;7:167-77. [Crossref] [PubMed]
- Skoglund K, Svensson G, Thilén U, et al. RV to PA conduits: impact of transcatheter pulmonary valve replacement in adults - a national register study. Scand Cardiovasc J 2017;51:153-8. [Crossref] [PubMed]
- Bacha EA, Kreutzer J. Comprehensive management of branch pulmonary artery stenosis. J Interv Cardiol 2001;14:367-75. [Crossref] [PubMed]
- Skoglund K, Svensson G, Thilén U, et al. Long-term outcome after right ventricle to pulmonary artery conduit surgery and reintervention. Scand Cardiovasc J 2017;51:284-91. [Crossref] [PubMed]
- Schwartzman WE, Jimenez M, Yates AR, et al. Patch Materials for Pulmonary Artery Arterioplasty and Right Ventricular Outflow Tract Augmentation: A Review. Pediatr Cardiol 2023;44:973-95. [Crossref] [PubMed]
- Powell AJ, Lock JE, Keane JF, et al. Prolongation of RV-PA conduit life span by percutaneous stent implantation. Intermediate-term results. Circulation 1995;92:3282-8. [Crossref] [PubMed]
- Peng LF, McElhinney DB, Nugent AW, et al. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation 2006;113:2598-605. [Crossref] [PubMed]
- Helal AM, Baho HA, Elmahrouk AF, et al. PR and QRS interval changes after transcatheter pulmonary valve replacement in children. Egypt Heart J 2023;75:66. [Crossref] [PubMed]


