HDL function and subclinical atherosclerosis in juvenile idiopathic arthritis
Introduction
Considerable evidence suggests that high-density lipoproteins (HDL) should protect against the development of atherosclerotic cardiovascular disease (1,2). In addition to playing a pivotal role in reverse cholesterol transport, HDL has been demonstrated to exert beneficial effects on inflammatory and oxidative pathways implicated in atherosclerosis (3). These functional activities are likely to underscore the observations that targeting HDL has an atheroprotective role in animal studies (4-6) and infusing lipid-deplete forms of HDL promotes rapid plaque regression in patients with a recent acute coronary syndrome (7). However, disappointing results of clinical trials of HDL raising agents (8-11) and a lack of association between genetic polymorphisms regulating HDL cholesterol levels and cardiovascular risk (12,13) have dampened enthusiasm for the HDL hypothesis.
In parallel, increasing attention has focused on the heterogeneity of circulating HDL particles and the potential implications for its functional quality. HDL has been demonstrated to vary in terms of its size, shape and composition of both lipid and protein. While some investigators have reported differences in HDL function according to different subspecies (14-16), the ultimate impact of this diversity on HDL functionality is uncertain. Several factors including inflammation, oxidative stress and hyperglycemia have each been demonstrated to impair the biological activities of HDL in the laboratory setting (17-19). This may imply that HDL is less protective in the setting of poorly controlled diabetes and inflammatory states, such as acute coronary syndromes.
Chronic inflammatory states, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) have each been observed to independently associate with an increased risk of cardiovascular disease in adults, after controlling for metabolic risk factors (20-22). While this may reflect the direct inflammatory effects on the artery wall, the impact of chronic inflammation on metabolic risk factors and lipoprotein function has not been well characterized. Studies of adults with SLE and RA have demonstrated impairment in HDL mediated cholesterol efflux capacity (23). The relative contribution that this functional impairment plays in the heightened cardiovascular risk is unknown.
Use of noninvasive arterial imaging has highlighted an increasing prevalence of early stages of atherosclerotic disease in adolescence (24-26). This is largely attributed to the rise in childhood obesity and its associated metabolic complications. Whether the presence of early chronic inflammatory states increases vascular risk has not been well studied. Juvenile idiopathic arthritis (JIA) describes a heterogeneous group of arthritis conditions that begin before age 16. While each disease entity has distinct clinical features, all conditions are characterized by systemic inflammation developing early in life and persisting for decades. The inflammatory cytokine milieu in JIA patients associates with higher blood pressure (27,28), dyslipidemia (29,30), intimal medial thickening (31), aortic stiffness (32), vascular dysfunction and increased CVD risk (33). It is therefore reasonable to speculate that cardiovascular risk may be increased in this population, potentially at younger ages than the general population. While HDL cholesterol levels are often lower in JIA patients (29,34,35), HDL functionality has not been studied in this population. The objective of the current cross-sectional case-control study was to characterize HDL functionality and the presence of subclinical atherosclerosis in a cohort of young patients with JIA.
Methods
Patient cohort
We conducted a prospective cross-sectional matched characteristic study of 44 patients, comprising 29 adolescents and young adults with JIA and 15 controls. The disease group were aged 10-35 years, fulfilled the diagnostic criteria of JIA according to the International League of Associations for Rheumatology (ILAR) criteria (36), and had the ability for either the subject or legal guardian to give informed consent and answer questions about past medical history and present symptoms. The control group was of a similar age, without any chronic inflammatory conditions and were able to provide informed consent, either directly or via their guardians. The presence of any other chronic inflammatory condition, diabetes mellitus, hypertension, dyslipidemia and an established history of cardiovascular disease excluded participants from the study.
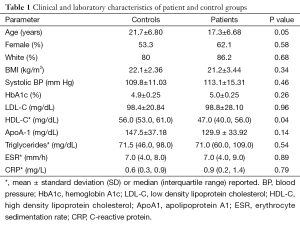
Demographics, medical history, medication use and details of JIA, including duration, subtype and systemic biomarker levels were recorded during the first visit. Limited physical examination recorded vital signs, height, weight and joint count. Within four weeks of the initial visit, blood was sampled for examination of lipid profile, uric acid, glucose, hemoglobin A1c and inflammatory markers. Characteristics of our cohort are listed in Table 1.
Full table
Cholesterol efflux
We characterized ATP binding cassette A-1 (ABCA1), ATP binding cassette G-1 (ABCG1), and scavenger receptor B-1 (SR-B1) mediated cholesterol efflux using apolipoprotein B depleted serum collected from patients and controls. Polyethylene glycol (PEG) precipitation of serum apoB containing lipoproteins was performed as previously described (14). ABCA1, ABCG and SR-B1 mediated efflux were measured as previously described using J774 macrophages, BHK cells, and Fu5AH rat hepatoma cells, respectively (14,37,38). Briefly, cells were plated and radiolabelled with [3H] cholesterol. ABCA1 and ABCG1 expression were upregulated using cAMP and mifepristone, respectively. Efflux medium with 2.8% apo-B depleted serum was incubated with cells for 4 hours. All samples were run in triplicate. Liquid scintillography was used to quantify efflux of radioactive cholesterol from cells. Cholesterol efflux capacity was calculated as ratio of average percentage efflux for each sample divided by average percentage efflux for control.
Cholesterol efflux receptor expression
Expression of ABCA1, ABCG1 and SR-B1 in response to HDL isolated by sequential ultracentrifugation from 10 patients and 10 controls with the most divergent CRP values. Murine bone marrow precursors were isolated from 8-week old male mice, differentiated into bone marrow macrophages for 5-10 days and exposed to HDL (50 µg apoA-I) from patients in duplicate. After mRNA isolation (RNeasy Mini Kit—Qiagen, Catalog # 74104, 74106) and cDNA synthesis, RT-PCR was performed in triplicate for each sample per gene (ABCA1, ABCG1, SR-B1) and for the internal control gene (18S). The comparative CT method (39) used to calculate the fold induction of each receptor.
HDL particle size measurement
HDL particle size was determined by nuclear magnetic resonance spectroscopy on plasma samples (LipoScience, Raleigh, NC, USA). Standard criteria for particle size measurements were used on the basis of their diameter (large HDL 8.8–13 nm, medium HDL 8.2–8.8 nm and small HDL 7.3–8.2 nm).
HDL remodelling by CETP
Cholesteryl ester transfer protein (CETP) facilitates the exchange of cholesteryl esters and triglycerides between lipoproteins. CETP activity was measured using an isotopic cholesteryl ester transfer assay similar to that previously described (40,41) with the use of radiolabelled LDL as donor lipoprotein and HDL as acceptor lipoprotein in the presence of serum from patients and controls. Samples were run in duplicate and incubated for 4.5 hours. After liquid scintillation counting, CETP activity was expressed as percentage of radioactivity transferred from [3H]-LDL to HDL after correction for blanks.
Antioxidant properties by PON-1
Antioxidant properties of HDL in patients and controls were measured by assessing paroxonase-1 (PON1) function using an arylesterase assay as previously described (42). Briefly, initial hydrolysis rates at 25 °C of phenyl acetate substrate (3.6 mmol/L) were determined at 270 nm in 1:50 diluted serum in reaction mixtures consisting of Tris hydrochloride 9 mmol/L, pH 8.0, and calcium chloride 0.9 mmol/L in a 96-well plate format (Spectramax 384 Plus; Molecular Devices). An extinction coefficient (at 270 nm) of 1,310 mol/L per cm was used for calculating units of arylesterase activity in serum, which are expressed as the amount of phenyl acetate hydrolyzed in units of µmol (L/min) per mL. All samples were run in duplicate and any sample with CV greater than 10 in the duplicates was repeated to ensure accuracy.
Vascular repair by endothelial cell migration
Endothelial cell migration across 8.0 µM Transwells was performed similar to previously described (43,44). Briefly, human umbilical vein endothelial cells (HUVEC) were seeded in the upper chamber and were allowed to migrate overnight at 37 °C in response to 75 µg HDL from each patient and control in the bottom chamber. All samples were run in duplicate. Migrated HUVECs were fixed and number of migrated cells was quantified after fluorescent labelling.
Carotid ultrasound
Carotid artery imaging was acquired following a standardized protocol using duplex ultrasound to enable measurement of carotid intima-medial thickness (cIMT) of the common carotid and bulb segments of the right and left carotid arteries. Images were stored digitally and analyzed in the Atherosclerosis Imaging Core Laboratory at the Cleveland Clinic by trained personnel, who were blinded to disease status of participants, for measurement of far wall cIMT using auto-edge detection software. The distance between the lumen-intima interface and the media-adventitia interface were measured at the bulb and distal common carotid artery. All measurements were confirmed by a vascular medicine physician.
Statistical analysis
Continuous variables were compared between patients and controls using the student’s t-test or Wilcoxon rank-sum test, with mean ± standard deviation (SD) or median [interquartile range (IQR)] reported depending on if the data was normally distributed or not, respectively. Analysis of variance (ANOVA) or Kruskal-Wallis tests were used in subgroup analyses with more than two groups, depending on if the data was normally distributed or not, respectively. Categorical variables were compared between treatment groups using the Pearson chi-square or Fisher’s exact test, with percentages reported. All tests were two-tailed with a 0.05 significance level. Analysis was performed using SAS version 9.2 (SAS Inc., Cary, NC, USA). Assuming a common SD of 0.15 and mean cIMT measurements of 0.45 mm in controls, the study had 80% power to detect a 0.05-mm in cIMT between the two groups using a 2-sided alpha of 0.05.
Results
Cohort characteristics
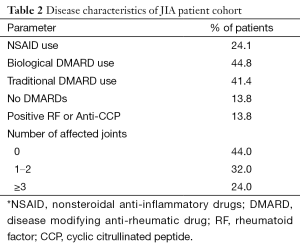
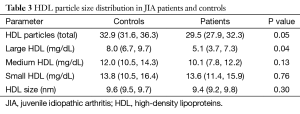
Table 1 summarizes the clinical characteristics of patients and controls. While JIA patients were aged 10–32 years and controls 10–33 years, JIA patients were significantly younger (17.3±6.7 vs. 21.7±6.8 years, P=0.05). HDL cholesterol was lower in JIA patients [47.0 (40.0, 56.0) vs. 56.0 (53.0, 61.0) mg/dL, P=0.04], consistent with prior reports. No other differences in demographics or cardiovascular risk factors were observed between patients and controls. Table 2 shows disease characteristics and medication use in the patient population. The majority of patients were treated with disease modifying anti-rheumatic drugs (DMARDs) (44.8% biological DMARDs, 41.4% traditional DMARDs). Despite treatment, more than half of JIA patients (56.0%) had persistent evidence of actively affected joints upon presentation. Table 3 shows HDL particle size distributions in patients and controls. Patients had less total HDL [29.5 (27.9, 32.3) vs. 32.9 (31.6, 36.3) mg/dL, P=0.05] and less large HDL [5.1 (3.7, 7.3) vs. 8.0 (6.7, 9.7) mg/dL, P=0.04] particles compared with controls.
Full table
Full table
Cholesterol efflux and receptor expression
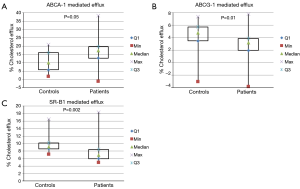
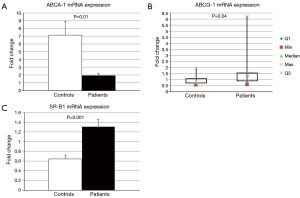
Figure 1 depicts ABCA1, ABCG1, and SR-B1 mediated cholesterol efflux with PEG precipitated serum from patients and controls. JIA patients had significantly higher ABCA1 [median (interquartile range), 17.3% (12.8, 19.7) vs. 10.0% (5.8, 16.0), P=0.05] and significantly lower ABCG1 [3.2% (2.0, 3.9) vs. 4.8% (3.5, 5.8), P=0.01] and SR-B1 [6.9% (6.0, 8.4) vs. 9.1% (8.6, 10.2), P=0.002] mediated efflux compared with controls. Figure 2 shows ABCA1, ABCG1 and SR-B1 mRNA expression of murine macrophages upon exposure to serum isolated from patients and controls. Patient serum resulted in smaller increases in macrophage expression of ABCA1 (2.0±0.95 vs. 7.1±5.7 fold increase, P=0.01) and greater increases in both ABCG1 [1.4 (0.9, 1.5) vs. 0.8 (0.7, 1.1) fold increase, P=0.04] and SR-B1 (1.3±0.47 vs. 0.7±0.3 fold increase, P=0.001) compared with controls.
Functional analyses
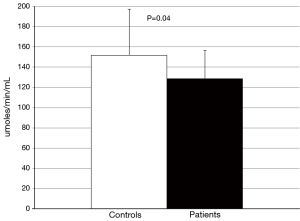
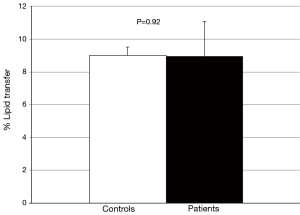
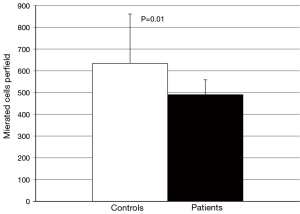
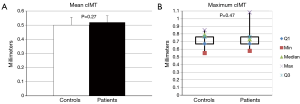
No difference in the ability of CETP to facilitate lipid transfer was observed between patients and controls [(9.0±1.8) vs. (9.0±2.1)% lipid transfer, P=0.92] (Figure 3). Arylesterase activity was lower in patients than controls (128.9±27.6 vs. 152.0±45.2 µmoles/min/mL, P=0.04) (Figure 4). Endothelial cell migration was lower upon exposure to patient (491.2±68.9 vs. 634.2±227.4 cells/field, P=0.01) (Figure 5). No difference was observed in mean cIMT (0.50±0.05 vs. 0.50±0.06 mm, P=0.27) or maximum cIMT [0.73 (0.67, 0.76) vs. 0.70 (0.67, 0.76) mm, P=0.47] between patients and controls respectively (Figure 6).
Discussion
Our analysis reveals that JIA has variable effects on HDL functionality in young people. We observed that JIA patients demonstrated lower levels of HDL-C in association with less circulating HDL particles, driven predominantly by a reduction in larger HDL particles. From a functional perspective, JIA was associated with less efflux mediated by ABCG1 and SR-B1, but greater ABCA1 mediated efflux compared with controls. Serum isolated from JIA patients was associated with less upregulation of macrophage expression of ABCA1 and greater expression of ABCG1 and SR-B1. Beyond effects on lipid transport factors, JIA patients demonstrated less antioxidant activity and impaired endothelial cell migration properties. These findings suggest that even in the setting of treatment for JIA, the presence of longstanding systemic inflammation is associated with altered HDL functionality.
Studies of HDL functionality in adults with chronic autoimmune diseases have demonstrated the presence of pro-inflammatory HDL with impaired anti-oxidant properties (45) and an inverse relationship between disease severity and cholesterol efflux capacity (46). Our findings that JIA patients demonstrate greater ABCA1 mediated efflux and less ABCG1 and SR-B1 mediated efflux is consistent with a shift in distribution of HDL particles from large to small, as demonstrated on NMR profiling. This change in HDL subparticle composition extends prior observations in JIA patients (29) to provide a functional context.
The etiology of the observed shift in HDL particle size distribution in JIA patients remains to be elucidated. Inflammation is likely to upregulate activity of lipolytic enzymes that will result in preferential accumulation of small lipid particles (17). Further investigations are required to determine the impact of chronic inflammatory diseases on factors influencing lipoprotein metabolism. Several groups have investigated the potential clinical implications of HDL particle size distribution on cardiovascular risk in adults, with consistent reports of an inverse relationship between large HDL particle concentrations and atherosclerotic disease (47). Imaging studies have demonstrated that levels of small HDL particles associate with subclinical measures of vascular disease, as measured by Cimt (48,49) in adults. In the current study, we did not observe a greater cIMT in younger individuals with JIA. Whether this reflects the lack of early changes in the artery wall or small sample size remains to be determined, but suggests further investigation is required in patients with early evidence of chronic inflammatory diseases.
In addition to upregulating lipolytic activity, inflammatory factors have been shown to influence cholesterol efflux mediators, with evidence that interferon-gamma (IFN-γ), interleukin 1-beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) downregulate ABCA1 expression on macrophages (50-52). Treatment strategies commonly employed in inflammatory arthropathies have been demonstrated to influence ABCA1 expression. COX-2 inhibitors and NSAIDs, which are commonly used by arthritis patients, downregulate macrophage ABCA1 expression (53). Methotrexate, which is commonly employed as the first line DMARD, attenuates the downregulation of ABCA1 by COX-2 inhibitors and IFN-γ (54). The effects of other DMARDs on receptor expression have not been studied, nor have there been studies on the effects of inflammation or medications on ABCG1 or SR-B1 expression. To our knowledge, our observations represent the first report that the presence of JIA associates with altered expression of not only ABCA1, but also both ABCG1 and SR-BI receptors. Whether these findings represent a direct effect on the inflammatory milieu or treatment regimen on receptor expression or simply a counter regulatory response to changes in acceptor/receptor interactions remains to be determined.
We also observed impairments beyond lipid transporting factors. The reduction in arylesterase activity in JIA patients is consistent with prior reports of decreased paraoxonase activity in adult patients with RA (46). This finding was complemented by the observation that serum from JIA patients was less effective in promoting endothelial cell migration, suggesting that there is early impairment of vascular repair activity with early inflammatory disease. How these observations translate to effects in the in vivo setting requires further investigation.
While this study is the first to demonstrate alterations in several aspects of HDL functionality in patients with JIA, certain limitations exist. The study has been conducted in a small cohort and the study design did not allow for longitudinal follow-up to determine effects of these changes in HDL functionality on clinical outcomes. Patients in our cohort had well controlled disease by inflammatory markers, though over half of them still had clinically active disease. A larger study would allow for recruitment of patients with more varied levels of disease. While cIMT is a validated surrogate for coronary atherosclerosis, it does not perfectly correlate with burden of coronary atherosclerosis. However, its non-invasive methodology make it a more logistically feasible modality of estimating atherosclerotic burden in this young population.
In summary, JIA patients demonstrated alterations in measures of cholesterol transport, antioxidant properties, and endothelial cell migration activities. These findings suggest the presence of early changes that may contribute to increases in cardiovascular risk. How these abnormalities translate to subclinical atherosclerosis and cardiovascular risk require further investigation in larger cohorts. Whether the presence of these abnormalities identify an individual who requires more aggressive cardiovascular risk modification or if they respond to intensification of anti-inflammatory therapy should be examined in future studies. If our results are validated in other cohorts, JIA patients represent a vulnerable population that should be more closely monitored for cardiovascular risk factor modification to reduce cardiovascular morbidity and mortality.
Acknowledgements
None.
Footnote
Conflicts of Interest: SJ Nicholls: Research Grant; Modest; Amgen, Inc., AstraZeneca Pharmaceuticals LP, Roche, Lilly USA, LLC, Resverlogix Corp, Novartis Pharmaceuticals Corporation. Consultant/Advisory Board; Modest; Takeda Pharmaceuticals North America, Inc, Roche, Merck & Co., Inc. The other authors have no conflicts of interest to declare.
References
- Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707-14. [PubMed]
- Castelli WP. Cholesterol and lipids in the risk of coronary artery disease--the Framingham Heart Study. Can J Cardiol 1988;4 Suppl A:5A-10A.
- Navab M, Reddy ST, Van Lenten BJ, et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 2011;8:222-32. [PubMed]
- Nicholls SJ, Dusting GJ, Cutri B, et al. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 2005;111:1543-50. [PubMed]
- Puranik R, Bao S, Nobecourt E, et al. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis 2008;196:240-7. [PubMed]
- Yuhanna IS, Zhu Y, Cox BE, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med 2001;7:853-7. [PubMed]
- Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292-300. [PubMed]
- AIM-HIGH Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255-67. [PubMed]
- HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203-12. [PubMed]
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109-22. [PubMed]
- Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089-99. [PubMed]
- Tsai MY, Johnson C, Kao WH, et al. Cholesteryl ester transfer protein genetic polymorphisms, HDL cholesterol, and subclinical cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2008;200:359-67. [PubMed]
- Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA 2008;299:2777-88. [PubMed]
- Asztalos BF, de la Llera-Moya M, Dallal GE, et al. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res 2005;46:2246-53. [PubMed]
- Favari E, Lee M, Calabresi L, et al. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J Biol Chem 2004;279:9930-6. [PubMed]
- Favari E, Calabresi L, Adorni MP, et al. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry 2009;48:11067-74. [PubMed]
- Tietge UJ, Maugeais C, Lund-Katz S, et al. Human secretory phospholipase A2 mediates decreased plasma levels of HDL cholesterol and apoA-I in response to inflammation in human apoA-I transgenic mice. Arterioscler Thromb Vasc Biol 2002;22:1213-8. [PubMed]
- Kalogerakis G, Baker AM, Christov S, et al. Oxidative stress and high-density lipoprotein function in Type I diabetes and end-stage renal disease. Clin Sci (Lond) 2005;108:497-506. [PubMed]
- Parikh NI, Gerschenson M, Bennett K, et al. Lipoprotein concentration, particle number, size and cholesterol efflux capacity are associated with mitochondrial oxidative stress and function in an HIV positive cohort. Atherosclerosis 2015;239:50-4. [PubMed]
- Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005;52:402-11. [PubMed]
- Naranjo A, Sokka T, Descalzo MA, et al. Cardiovascular disease in patients with rheumatoid arthritis: results from the QUEST-RA study. Arthritis Res Ther 2008;10:R30. [PubMed]
- Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011;13:R131. [PubMed]
- Ronda N, Favari E, Borghi MO, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis 2014;73:609-15. [PubMed]
- Oren A, Vos LE, Uiterwaal CS, et al. Change in body mass index from adolescence to young adulthood and increased carotid intima-media thickness at 28 years of age: the Atherosclerosis Risk in Young Adults study. Int J Obes Relat Metab Disord 2003;27:1383-90. [PubMed]
- Fang J, Zhang JP, Luo CX, et al. Carotid Intima-media thickness in childhood and adolescent obesity relations to abdominal obesity, high triglyceride level and insulin resistance. Int J Med Sci 2010;7:278-83. [PubMed]
- Kotb NA, Gaber R, Salama M, et al. Clinical and biochemical predictors of increased carotid intima-media thickness in overweight and obese adolescents with type 2 diabetes. Diab Vasc Dis Res 2012;9:35-41. [PubMed]
- Oguz D, Ocal B, Ertan U, et al. Left ventricular diastolic functions in juvenile rheumatoid arthritis. Pediatr Cardiol 2000;21:374-7. [PubMed]
- Alkady EA, Helmy HA, Mohamed-Hussein AA. Assessment of cardiac and pulmonary function in children with juvenile idiopathic arthritis. Rheumatol Int 2012;32:39-46. [PubMed]
- Tselepis AD, Elisaf M, Besis S, et al. Association of the inflammatory state in active juvenile rheumatoid arthritis with hypo-high-density lipoproteinemia and reduced lipoprotein-associated platelet-activating factor acetylhydrolase activity. Arthritis Rheum 1999;42:373-83. [PubMed]
- Bakkaloglu A, Kirel B, Ozen S, et al. Plasma lipids and lipoproteins in juvenile chronic arthritis. Clin Rheumatol 1996;15:341-5. [PubMed]
- Vlahos AP, Theocharis P, Bechlioulis A, et al. Changes in vascular function and structure in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:1736-44. [PubMed]
- Argyropoulou MI, Kiortsis DN, Daskas N, et al. Distensibility and pulse wave velocity of the thoracic aorta in patients with juvenile idiopathic arthritis: an MRI study. Clin Exp Rheumatol 2003;21:794-7. [PubMed]
- Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis--the next step: differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford) 2011;50:1944-54. [PubMed]
- Ilowite NT, Samuel P, Beseler L, et al. Dyslipoproteinemia in juvenile rheumatoid arthritis. J Pediatr 1989;114:823-6. [PubMed]
- Gonçalves M, D'Almeida V, Guerra-Shinohara EM, et al. Homocysteine and lipid profile in children with Juvenile Idiopathic Arthritis. Pediatr Rheumatol Online J 2007;5:2. [PubMed]
- Hochberg MC, Chang RW, Dwosh I, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992;35:498-502. [PubMed]
- Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127-35. [PubMed]
- Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res 2006;99:1031-43. [PubMed]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008;3:1101-8. [PubMed]
- Ahnadi CE, Berthezène F, Ponsin G. Simvastatin-induced decrease in the transfer of cholesterol esters from high density lipoproteins to very low and low density lipoproteins in normolipidemic subjects. Atherosclerosis 1993;99:219-28. [PubMed]
- Tato F, Vega GL, Grundy SM. Bimodal distribution of cholesteryl ester transfer protein activities in normotriglyceridemic men with low HDL cholesterol concentrations. Arterioscler Thromb Vasc Biol 1995;15:446-51. [PubMed]
- Tang WH, Hartiala J, Fan Y, et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler Thromb Vasc Biol 2012;32:2803-12. [PubMed]
- Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol 2008;28:2071-7. [PubMed]
- Leavesley DI, Schwartz MA, Rosenfeld M, et al. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol 1993;121:163-70. [PubMed]
- McMahon M, Grossman J, FitzGerald J, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 2006;54:2541-9. [PubMed]
- Charles-Schoeman C, Lee YY, Grijalva V, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis 2012;71:1157-62. [PubMed]
- Guey LT, Pullinger CR, Ishida BY, et al. Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am J Cardiol 2011;108:360-6. [PubMed]
- de Vries R, Perton FG, van Tol A, et al. Carotid intima media thickness is related positively to plasma pre ß-high density lipoproteins in non-diabetic subjects. Clin Chim Acta 2012;413:473-7. [PubMed]
- Hirayama S, Miida T, Miyazaki O, et al. Pre beta1-HDL concentration is a predictor of carotid atherosclerosis in type 2 diabetic patients. Diabetes Care 2007;30:1289-91. [PubMed]
- Alfaro Leon ML, Evans GF, Farmen MW, et al. Post-transcriptional regulation of macrophage ABCA1, an early response gene to IFN-gamma. Biochem Biophys Res Commun 2005;333:596-602. [PubMed]
- Hao XR, Cao DL, Hu YW, et al. IFN-gamma down-regulates ABCA1 expression by inhibiting LXRalpha in a JAK/STAT signaling pathway-dependent manner. Atherosclerosis 2009;203:417-28. [PubMed]
- Chen M, Li W, Wang N, Zhu Y, et al. ROS and NF-kappaB but not LXR mediate IL-1beta signaling for the downregulation of ATP-binding cassette transporter A1. Am J Physiol Cell Physiol 2007;292:C1493-501. [PubMed]
- Chan ES, Zhang H, Fernandez P, et al. Effect of cyclooxygenase inhibition on cholesterol efflux proteins and atheromatous foam cell transformation in THP-1 human macrophages: a possible mechanism for increased cardiovascular risk. Arthritis Res Ther 2007;9:R4. [PubMed]
- Reiss AB, Carsons SE, Anwar K, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum 2008;58:3675-83. [PubMed]