Overview of left ventricular outpouchings on cardiac magnetic resonance imaging
Introduction
Left ventricular diverticulum, aneurysms and the pseudoaneurysms are now diagnosed with increased frequency with the advent of recent imaging techniques. Patients may be totally asymptomatic or may have overlapping symptoms of heart failure, chest pain, or dyspnea as seen in both aneurysms and pseudoaneurysms. The physical examination findings and ECG results are also nonspecific (1-3). Magnetic resonance imaging (MRI) plays an important role in the characterization of these entities (Table 1). Wall motion abnormality can be demonstrated on cine MRI and the delayed enhancement corresponding to fibrosis is very well depicted on delayed enhanced MRI. Moreover there is no risk of radiation exposure using this non-invasive modality.
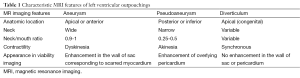
Full table
Cardiac MRI technique
Cardiac MRI is usually performed at our institution on a 1.5T scanner (Avanto, Siemens Healthcare) using a standard imaging protocol, with additional planes and sequences according to the specific clinical question. Black-blood-prepared half-Fourier single-shot turbo spin echo images [TR (ms)/TE (ms), 700/42; flip angle, 160°; thickness, 6 mm] are acquired in axial and coronal planes as contiguous sections, covering the thorax from the diaphragm to the thoracic inlet. Bright-blood-prepared balanced steady state free precession sequences [TR (ms)/TE (ms), 310/1.2; flip angle, 80°; thickness, 8 mm] are then used to quickly plan imaging along the cardiac axes. These are followed by fast spin-echo images with T1 weighting [TR (ms)/TE (ms), 700/30; flip angle, 180°; thickness, 6 mm] and T2-weighting with fat suppression [TR (ms)/TE (ms), 800/77; flip angle, 180°; thickness, 6 mm] along both four chamber and short-axis planes. Cine balanced steady state free precession sequences are then acquired in short-axis, two- and four-chamber planes. After this assessment, the patient is administered an intravenous injection of 0.2 mmol/kg of gadolinium-based contrast agent at 4 mL/s to acquire a perfusion scan using Turboflash sequence [TR (ms)/TE (ms), 178.90/1.05; flip angle, 15°; thickness, 10 mm]. A variable inversion time scout sequence is performed to select optimal inversion time to null normal myocardial signal. After 5 minutes of injection, post gadolinium images are optionally acquired according to the specific clinical indication. Late gadolinium enhancement images are acquired after 15 minutes of contrast injection in the short axis, two and four chamber planes using phase-sensitive inversion recovery sequence [TR (ms)/TE (ms), 700/1.22; flip angle, 45°; thickness, 8 mm].
Technical advancements in MRI
Recent technical advancements in the form of three-dimensional whole heart sequences allow 3-D reconstruction (4). Moreover, three-dimensional, prospective self-gating MRI with ECG-triggered, free-breathing respiratory gating as well as with simultaneous cardiac and respiratory compensation holds considerable promise for scanning patients who are unable to hold their breath or small children with reduced compliance under sedation during MRI examination (5,6).
Advanced sequences such as time-resolved angiography with interleaved stochastic trajectories (TWIST) allow for dynamic acquisition and with adding physiology to morphology. This reproduces adequate image quality with less amount of contrast material and is a promising method for dynamic contrast enhanced MRI (7). T1 mapping is another novel advanced MRI technique which has the potential to provide a quantitative assessment of diffuse myocardial fibrosis (8).
There is no associated risk of radiation exposure. Moreover, gadolinium based MRI contrast media show no cross reactivity with iodine-based contrast media due to its different chemical structure and can be a potential alternative in patients with iodine allergy or renal insufficiency (9). There is substantially reduced risk of severe allergic reaction occurring after gadolinium as compared to iodinated contrast. Also the risk of renal failure with the use of gadolinium is negligible. Nephrogenic systemic fibrosis is a rare complication occurring with high dose of certain extracellular gadolinium-based contrast media in patients with severely reduced renal function or those on dialysis (10). However, stable gadolinium-based contrast agents that have not been correlated to nephrogenic systemic fibrosis are also available.
Discussion
Distinction between various left ventricular outpouchings is of great concern as the management options vary considerably with different prognosis. Unfortunately, distinction between these entities may be difficult. Their characteristics on various imaging modalities show considerable overlap. Pathologically a true aneurysm contains elements of myocardium, whereas a pseudoaneurysm does not, while the diverticulum contains all the myocardial layers. Thrombus lining a pseudoaneurysm cavity can complicate the diagnosis and its differentiation from the myocardium becomes difficult on most of the conventional imaging techniques. MRI is very helpful in tissue characterization and can distinguish between pericardium, thrombus, and the myocardium. However, this characterization on conventional MRI can be difficult in thinned out and scarred infracted myocardium. Viability imaging using delayed enhanced MRI accurately delineates the area of fibrous scarring and wall motion abnormality is better seen on cine imaging. Cardiac MRI also has an important role in planning the surgical approach as it gives valuable information about the relation of the defect to the mitral valve and the potential viability of the myocardium in the non-aneurysmal portion of the left ventricle (11). Moreover reversible myocardial dysfunction identified by gadolinium enhanced MRI strongly correlates with the likelihood of improvement in contractility following revascularization (12). Echocardiography is helpful in the diagnostic work up and remains the initial test of choice, however it is operator dependent and is sometimes limited in the detection of these lesions in echocardiographically “silent” zones such as anterobasal and apical regions. Moreover, there may be occasional failure to adequately characterize the neck of the lesion. Multi-detector computed tomography and conventional angiography involves radiation exposure, besides invasiveness of conventional angiography procedure.
Left ventricular aneurysm
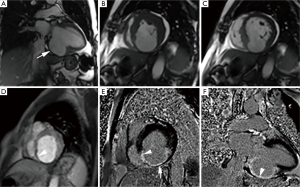
Left ventricular aneurysm represents an outpouching containing endocardium, epicardium and thinned, abnormally contracting and scarred myocardium which show characteristic constant functional wall motion abnormality, usually dyskinesia. They are more common in an apical, anterior, or anterolateral location and usually arise from an anterior descending vascular territory infarction (13). During surgery, it appears flaccid and puckered after left ventricle is evacuated. Dense collagenous or hyaline connective tissue, occasionally interspersed with a few muscle fibres surrounded by scarring, and foci of mononuclear cells are usually seen on histopathologic examination (14). Transmural myocardial infarction is the most common underlying cause; however trauma, iatrogenic injury, Chagas disease, hypertrophic cardiomyopathy, mucopolysaccharidosis, myocarditis, vasculitis and sarcoidosis are other less common reported causes (15-19). Their incidence has decreased significantly due to improvements in the treatment of acute myocardial infarction as shown in one report from 18.8% to 7.2% (20). Distinctive morphologic features on MRI (Figure 1) include wide neck and smooth transition from normal myocardium to thinned myocardium. The ratio of the maximum diameter of the orifice to the maximum internal diameter of the cavity is 0.9-1.0 for true aneurysms (21). Dyskinesia is well seen on cine MRI (Figure 1B,C). Thinned out, fibrous scarred area is well demonstrated on delayed enhanced MRI without pericardial enhancement (Figure 1E,F). Fatty replacement of the infarcted myocardial wall, calcification, and thrombus formation may be seen in chronic aneurysms. True aneurysm carries lower risk of rupture than a pseudoaneurysm and is often managed medically with surgical repair typically reserved in cases with associated heart failure, systemic embolization or refractory arrhythmia (22).
Left ventricular pseudoaneurysm
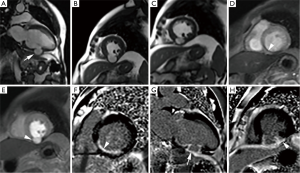
It is defined as an outpouching that results from myocardial rupture which is contained locally by thrombus and pericardial adhesions without a myocardial layer. Pseudoaneurysm most often results from myocardial infarction commonly following circumflex coronary artery occlusion however, it may also occur after cardiac surgery, trauma or infection (23,24). Morphologically, pseudoaneurysms are characterized by a narrow-necked channel that connects the large aneurysmal sac with the ventricle. Pseudoaneurysms more commonly involve posterior or inferior walls. Infarction at this location is often large and commonly associated with acute mitral regurgitation and high mortality. Color Doppler usually shows turbulent flow at the opening while prolonged retention of the intravascular contrast with narrow neck is the usual angiographic characteristics of pseudoaneurysms. The ratio of the maximum diameter of the orifice to the maximum internal diameter of the cavity is between 0.25 and 0.5 for pseudoaneurysms (21). The presence of a globular cavity, a narrow neck, abrupt transition from normal myocardium to aneurysm, and a distinct discontinuity of the ventricular wall are characteristic of pseudoaneurysms. Obliteration of the myocardial-pericardial interface and the delayed enhancement of the overlying pericardium is very useful clue to the diagnosis of pseudoaneurysm (25). These features are well seen on MRI (Figure 2). This enhancement is hypothesized to be due to chemical irritation of the pericardium by blood released in the acute phase of the myocardial rupture which induces an inflammatory reaction and pericardial neovascularisation. Pseudoaneurysms are prone to rupture as they lack normal myocardium which necessitates urgent surgical repair.
Left ventricular diverticulum
Left ventricular diverticulum is an outpouching that contains endocardium, myocardium, and pericardium and displays synchronous contractility. Earlier studies reported a prevalence of 0.4%, however increased prevalence of 2.2% is seen in one study using multi-detector computed tomography angiography (26). They may be congenital or acquired, former being more common. In utero viral infection, muscle and connective-tissue defects, and excessive primordial cell stimulation are proposed as causes of congenital diverticula. Mostly they are found in the apical region. Apical diverticula are usually associated with midline thoracoabdominal defects and other heart malformations, whereas non-apical diverticula are isolated (27). Cantrell syndrome represents syndromic association of left ventricular diverticulum with midline thoracoabdominal congenital abnormalities, diaphragmatic and sternal defects, and partial absence of pericardium (28). These diverticula are often asymptomatic and may be found incidentally during diagnostic work up for other reasons. Their size may vary from as small as 0.5 cm in diameter to as large as 8-9 cm (29). They show synchronous contractility and small diverticula usually closes during systole (26). Their connection to the ventricle may be narrow or wide. On MRI (Figure 3), normal myocardial signal intensity is seen in its wall without any discontinuity or enhancement on post gadolinium sequences. On cine MRI, they show synchronous contractility. Thrombosis, embolism, rupture and ventricular arrhythmias can complicate these diverticula, however true incidence of these complications is not known. Spontaneous regression is seen in few cases and their size may not change over a considerable period of time suggesting a benign course (30). Close clinical follow up is usually sufficient and further management should be based on associated abnormalities and potential complications.
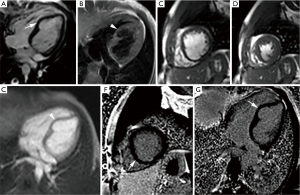
Myocardial crypts constitute an entity with uncertain clinical significance and sometimes need to be differentiated from ventricular diverticulum. It is usually seen as a narrow, deep blood-filled invagination in otherwise compacted left ventricular myocardium. Most of the definitions used a penetration of more than 50% of the myocardial thickness in diastole as one of the criteria for its diagnosis (31). These crypts are perpendicular to the endocardial border of otherwise normal compacted myocardium and show subtotal or total obliteration during systole. They commonly involve basal inferoseptal region and can be well seen on routine two chamber view. However a modified imaging plane through the inferoseptum further improves their identification. They are relatively common in the normal population with overall prevalence of 6-7% (31,32). They are increasingly common in hypertrophic and hypertensive cardiomyopathy. Moreover, the presence of a crypt has also been suggested as a predictor of gene carrier status in hypertrophic cardiomyopathy (33,34).
Conclusions
Accurate diagnosis of left ventricular outpouchings is of prime importance as their prognosis varies widely from benign to catastrophic. Cardiac MRI with its advanced sequences can characterize these outpouchings very well, thus helping clinicians to better understand their natural history and to guide proper management decisions with accurate diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heatlie GJ, Mohiaddin R. Left ventricular aneurysm: comprehensive assessment of morphology, structure and thrombus using cardiovascular magnetic resonance. Clin Radiol 2005;60:687-92. [PubMed]
- Cho MN, Mehta SK, Matulevicius S, et al. Differentiating true versus pseudo left ventricular aneurysm: a case report and review of diagnostic strategies. Cardiol Rev 2006;14:e27-30. [PubMed]
- Frances CD, Shlipak MG, Grady D. Left ventricular pseudoaneurysm: diagnosis by cine magnetic resonance imaging. Cardiology 1999;92:217-9. [PubMed]
- Uribe S, Muthurangu V, Boubertakh R, et al. Whole-heart cine MRI using real-time respiratory self-gating. Magn Reson Med 2007;57:606-13. [PubMed]
- Buehrer M, Curcic J, Boesiger P, et al. Prospective self-gating for simultaneous compensation of cardiac and respiratory motion. Magn Reson Med 2008;60:683-90. [PubMed]
- Manka R, Buehrer M, Boesiger P, et al. Performance of simultaneous cardiac-respiratory self-gated three-dimensional MR imaging of the heart: initial experience. Radiology 2010;255:909-16. [PubMed]
- Le Y, Kroeker R, Kipfer HD, et al. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging 2012;36:483-91. [PubMed]
- Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson 2014;16:2. [PubMed]
- Saleh L, Juneman E, Movahed MR. The use of gadolinium in patients with contrast allergy or renal failure requiring coronary angiography, coronary intervention, or vascular procedure. Catheter Cardiovasc Interv 2011;78:747-54. [PubMed]
- Thomsen HS, Marckmann P, Logager VB. Enhanced computed tomography or magnetic resonance imaging: a choice between contrast medium-induced nephropathy and nephrogenic systemic fibrosis? Acta Radiol 2007;48:593-6. [PubMed]
- Dutta R, Kahn AM, Rathod A, et al. Utility of cardiac magnetic resonance imaging in evaluating left ventricular pseudoaneurysm. Congest Heart Fail 2008;14:91-4. [PubMed]
- Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445-53. [PubMed]
- Higgins CB, Lipton MJ, Johnson AD, et al. False aneurysms of the left ventricle. Identification of distinctive clinical, radiographic, and angiographic features. Radiology 1978;127:21-7. [PubMed]
- Lillehei CW, Levy MJ, Dewall RA, et al. Resection of myocardial aneurysms after infarction during temporary cardiopulmonary bypass. Circulation 1962;26:206-17. [PubMed]
- Frustaci A, Chimenti C, Pieroni M. Prognostic significance of left ventricular aneurysms with normal global function caused by myocarditis. Chest 2000;118:1696-702. [PubMed]
- Studer MA, Jefferies JL, McKenzie ED, et al. Traumatic cardiac rupture and left ventricular aneurismal formation in childhood. Am J Cardiol 2008;101:413-4. [PubMed]
- Paul M, Schäfers M, Grude M, et al. Idiopathic left ventricular aneurysm and sudden cardiac death in young adults. Europace 2006;8:607-12. [PubMed]
- De La Calzada CS, Verdugo AL, Perez JT, et al. Hypertrophic cardiomyopathy associated with left ventricular aneurysm and normal coronary arteries: Case study indicating genetic tendencies of cardiomyopathy. Cardiovasc Dis 1981;8:73-83. [PubMed]
- Oudit GY, Butany J, Williams WG, et al. Images in cardiovascular medicine. Left ventricular aneurysm associated with mucopolysaccharidosis type VI syndrome (Maroteaux-Lamy syndrome). Circulation 2007;115:e60-2. [PubMed]
- Tikiz H, Balbay Y, Atak R, et al. The effect of thrombolytic therapy on left ventricular aneurysm formation in acute myocardial infarction: relationship to successful reperfusion and vessel patency. Clin Cardiol 2001;24:656-62. [PubMed]
- Gatewood RP Jr, Nanda NC. Differentiation of left ventricular pseudoaneurysm from true aneurysm with two dimensional echocardiography. Am J Cardiol 1980;46:869-78. [PubMed]
- Lundblad R, Abdelnoor M, Svennevig JL. Repair of left ventricular aneurysm: surgical risk and long-term survival. Ann Thorac Surg 2003;76:719-25. [PubMed]
- Kollar A, Byrd BF 3rd, Lui HK, et al. Mitral valve replacement and endocavitary patch repair for a giant left ventricular pseudoaneurysm. Ann Thorac Surg 2001;71:2020-2. [PubMed]
- Bauer M, Musci M, Pasic M, et al. Surgical treatment of a chest-wall penetrating left ventricular pseudoaneurysm. Ann Thorac Surg 2000;70:275-6. [PubMed]
- Konen E, Merchant N, Gutierrez C, et al. True versus false left ventricular aneurysm: differentiation with MR imaging--initial experience. Radiology 2005;236:65-70. [PubMed]
- Srichai MB, Hecht EM, Kim DC, et al. Ventricular diverticula on cardiac CT: more common than previously thought. AJR Am J Roentgenol 2007;189:204-8. [PubMed]
- Marijon E, Ou P, Fermont L, et al. Diagnosis and outcome in congenital ventricular diverticulum and aneurysm. J Thorac Cardiovasc Surg 2006;131:433-7. [PubMed]
- Manieri S, Adurno G, Iorio F, et al. Cantrell's Syndrome with left ventricular diverticulum: a case report. Minerva Pediatr 2013;65:93-6. [PubMed]
- Ohlow MA. Congenital left ventricular aneurysms and diverticula: definition, pathophysiology, clinical relevance and treatment. Cardiology 2006;106:63-72. [PubMed]
- Archbold RA, Robinson NM, Mills PG. Long-term follow-up of a true contractile left ventricular diverticulum. Am J Cardiol 1999;83:810-3, A11.
- Child N, Muhr T, Sammut E, et al. Prevalence of myocardial crypts in a large retrospective cohort study by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2014;16:66. [PubMed]
- Petryka J, Baksi AJ, Prasad SK, et al. Prevalence of inferobasal myocardial crypts among patients referred for cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2014;7:259-64. [PubMed]
- Rowin EJ, Maron MS. Myocardial crypts in hypertrophic cardiomyopathy: the new gang in town. Eur Heart J Cardiovasc Imaging 2012;13:281-3. [PubMed]
- Brouwer WP, Germans T, Head MC, et al. Multiple myocardial crypts on modified long-axis view are a specific finding in pre-hypertrophic HCM mutation carriers. Eur Heart J Cardiovasc Imaging 2012;13:292-7. [PubMed]


