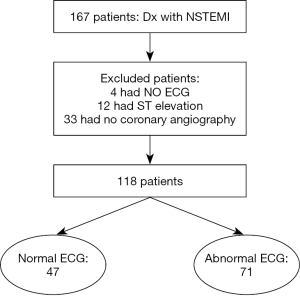
Anatomic distribution of culprit lesions in patients with non-ST-segment elevation myocardial infarction and normal ECG
Introduction
Non-ST-elevation myocardial infarction (NSTEMI) is a leading cause of morbidity and mortality (1-3). Although the electrocardiogram on admission is often abnormal, the presence of normal ECG does not exclude disease (4,5). A left circumflex (LCX) coronary artery occlusion may be present with normal ECG (6). Risk assessment is a cornerstone in evaluation of NSTEMI patients. While ST depression is one of seven factors that predict higher mortality in NSTEMI patients (TIMI score) (7), there are less data with those with no ST depression and normal ECG on presentation. For patients presenting with NSTEMI to a tertiary referral center in the Middle East, we sought to compare the characteristics of culprit lesions of those with normal versus abnormal ECG, short-term clinical endpoints, and assess the predictors of culprit lesion using ECG, echocardiographic and clinical parameters.
Methods
Patient selection
From the coronary cardiac unit database at the American University of Beirut Medical Center (AUBMC), we identified consecutive patients with the diagnosis of NSTEMI that were admitted between June 2012 and December 2013. NSTEMI was defined according to the ACC/AHA guidelines (8) and the universal definition of myocardial infarction (9) as the presence of positive cardiac troponin with levels greater than 99th percentile upper reference limit AND either symptoms suggestive of ischemia, new ST/TW changes (ST elevation was excluded), new Q waves, or new wall motion abnormalities on echocardiogram. Of a total of167 patients, 49 patients were excluded (33 did not undergo coronary angiography, 4 had missing electrocardiogram, and 12 had ST elevation on the admission electrocardiogram after careful review), leaving 118 for final analysis (Figure 1). Patients demographics, co-morbidities, medications at admission and discharge, baseline laboratory values, admission electrocardiogram, echocardiogram and coronary angiography results, were extracted from chart review. The study was approved by the Institutional Review Board at AUBMC with waiver of informed consent.
ECG interpretation
The admission 12-leads electrocardiograms for each patient were extracted for review. A board certified electrophysiologist (BA) that was blinded to the coronary angiography results, interpreted each electrocardiogram and recorded the presence or absence of ST segment depression, T wave changes, Q waves, or bundle branch block. The electrocardiographic results were then dichotomized as either normal (none of the above changes were present) or abnormal (one or more of the above findings were observed).
Echocardiography
Baseline echocardiography was performed on admission or after the coronary angiogram in the lateral decubitus position, in a standard fashion using commercially available systems (Philips Electronics, Andovers, MA, USA; GE Medical Systems, Milwaukee, WI, USA; Siemens Medical Solutions, MountainView, CA, USA). Echocardiographic parameters such as left ventricular (LV) end-diastolic and systolic dimensions, LV mass, ejection fraction (EF), left atrial volume index, and diastolic function were extracted from the echocardiography report. The images were reviewed for the presence or absence of wall motion abnormalities. LV EF was assessed by semi-quantitative manner using the Biplane Simpson method, and left atrial volume index in accordance with published guidelines (10). Diastolic function was assessed in our institution in a standardized method and in accordance with the most recently published guidelines by American Society of Echocardiography using a combination of echocardiographic variables (transmitral inflow pattern, mitral annular velocities with tissue Doppler imaging, left atrial volume index, and pulmonary venous flow pattern (11). Diastolic function was labeled as normal or abnormal (DD); DD was then categorized as mild (grade 1, impaired relaxation),moderate (grade 2, pseudonormal), or severe (grade 3, restrictive).
Coronary angiography
The coronary angiography films and reports were reviewed. The presence or absence of culprit lesion, number of lesions, location and segment involved, degree of initial stenosis, type of intervention, and residual stenosis were extracted. The location and segment involved were coded according to the SCCT coronary segments diagram (12). The degree of stenosis was visually estimated or semi-quantified by the interventionalist at the time of the procedure and retrieved from chart review. All co-authors were blinded to the results of the ECG during data extraction.
Endpoints
The primary endpoint was an abnormal coronary angiogram or presence of a culprit lesion. Secondary endpoints included length of stay, re-hospitalization within 60 days, and in-hospital mortality.
Statistical analysis
Continuous variables were expressed as means ± SD and compared by use of the unpaired Student t test or Wilcoxon rank test as appropriate. Categorical variables were expressed as percentages and compared by use of the Fisher exact test or Pearson Chi-square test as appropriate. Comparisons between variables at baseline versus follow-up were made with the Paired t test (continuous) or McNemar test (categorical). Multivariate regression analysis model was performed to determine the independent predictors of an abnormal coronary angiogram. Clinically relevant variables such as age, co-morbidities (diabetes, history of CAD), EF and wall motion abnormalities, cardiac enzymes, and admission ECG were entered into the model using stepwise forward selection. Variables with collinearity were entered into the model one at a time. All statistical tests were 2 sided. A P value
Result
Patient characteristics
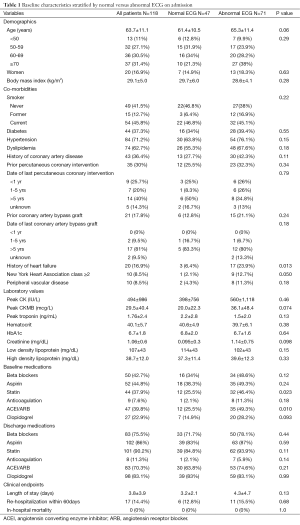
A total of 118 patients (mean age 63.7±11.1 years, 83% male) were identified and analyzed. There was significant prevalence of cardiovascular risk factors including diabetes mellitus (37%), hypertension (73%), and smoking history (59%), with more than one third of patients having known prior coronary artery disease (CAD) (Table 1).
Full table
Electrocardiographic results
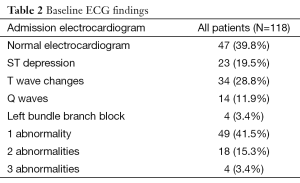
There were 47 (39.8%) patients with normal and 71 (60.2%) with abnormal ECG. The most common ECG abnormalities were T wave changes, followed by ST depression (Table 2). Patients with normal ECG were younger, had lower prevalence of known CAD, heart failure, intake of cardiovascular medications, lower creatinine and peak CKMB (Table 1).
Full table
Echocardiographic results
Baseline echocardiography was performed on all patients. The mean LVEF was (50±13)% with 20% having EF Table 3).

Full table
Coronary angiography
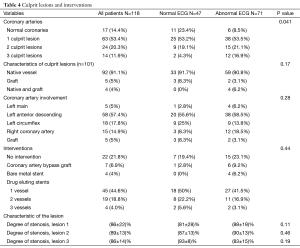
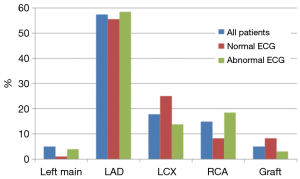
A total of 101 patients (86%) had abnormal coronary angiogram; 47% had more than one culprit lesion, and 57% had a culprit lesion in the left anterior descending (LAD) coronary artery. The majority underwent subsequent revascularization with angioplasty and drug eluting stents (only 4 received bare metal stents), 7 (7%) underwent CABG and 22 (22%) had no intervention (Table 4). A total of 17 patients had normal coronary angiogram; two had confirmed myocarditis by cardiac magnetic resonance imaging, two had severe aortic stenosis, one had takatsubo cardiomyopathy, and one had septic shock. The remaining patients had no clear documented diagnosis.
Full table
Patients with normal ECG had significantly more normal coronary angiogram (23% vs. 8.5%, P=0.04), but similar distribution of culprit lesion, interventions, and degree of stenosis (Table 4). However, there was a trend toward more involvement of the LCX coronary artery (25% vs. 13.8%, P=0.18) (Figure 2).
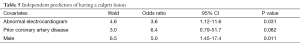
After adjusting for age, co-morbidities (diabetes, history of CAD), EF, wall motion abnormalities, cardiac enzymes, and admission ECG, multivariate regression analysis showed that prior history of CAD [odds ratio (OR) 6.4 (0.8-52)], male gender [OR 5.0 (1.5-17)], and abnormal ECG [OR 3.6 (1.12-12)] were independent predictors of the presence of a culprit lesion (Table 5). The sensitivity, specificity, negative and positive predictive value of ECG to detect a culprit lesion in patients with NSTEMI were: 64%; 65%; 23%; and 92%, respectively.
Full table
The mean length of hospital stay was 3.8±3.9 days and was not significantly different between those with normal versus abnormal ECG. There was no in-hospital mortality and the rate of re-hospitalization was 14.4% for all patients and similar between groups. Patients with culprit lesions had similar length of stay but had a trend toward more hospitalization (15.8% vs. 5.9%, P=0.28).
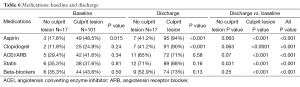
The admission and discharge cardiovascular medications, stratified by patients with and without culprit lesions, are summarized in Table 6. A significantly higher percentage of patients with culprit lesions was discharged on cardiovascular medications as compared to those with normal coronaries, although significantly higher than at admission for both groups. There was no difference in cardiac medications when stratified by normal versus abnormal ECG (Table 1).
Full table
Discussion
In the current single center retrospective study of patients with NSTEMI presenting to a tertiary referral center in the Middle East, we found that: (I) 86% of patients had a culprit lesion, most commonly the LAD; (II) those with normal ECG had more normal coronary arteries or LCX involvement; (III) there was no difference in length of stay or re-hospitalization and no in-hospital mortality between groups; (IV) an abnormal ECG increased the odds of a culprit lesion by more than 3 folds.
A normal ECG in patients presenting with NSTEMI does not characterize a particular culprit lesion. Indeed, the LAD was the most commonly encountered culprit lesion in those with normal and abnormal ECG, and without predilection for proximal, mid or distal lesions. However, those with normal ECG had a higher trend of having LCX involvement, emphasizing therefore the importance of posterior ECG leads (V7-9) (13-15) which became standard of care in patients whose initial ECG is non diagnostic and who are at intermediate/high risk of acute coronary syndrome (class IIa, Level of evidence B) (16). It is estimated that 4% of all acute myocardial infarction reveal the presence of isolated ST elevation in posterior chest leads (15,17). Furthermore, patients with normal ECG had significantly higher percentage of normal coronary angiogram (23% vs. 8.5%, P=0.04). Several medical conditions can result in elevated troponin in absence of CAD on coronary angiogram. Of the 17 patients with normal angiogram, only 2 underwent cardiac MRI and were diagnosed with myocarditis. The most recent imaging guidelines recommend the use of cardiac MRI in patients with positive cardiac enzymes and normal coronary arteries to rule out myocarditis (18,19).
There was no significant difference in length of stay, re-hospitalization or in-hospital mortality between those with normal and abnormal ECG, or between those with or without culprit lesion. This can be attributed to the prompt administration of medical therapy and coronary intervention for all NSTEMI patients regardless of the initial ECG result, and also the small sample size.
On the other hand and not surprisingly, patients with NSTEMI and abnormal ECG had more wall motion abnormality, lower LVEF, worse diastolic function (Table 3), and a significantly higher number of two and three vessel disease (Table 4). An abnormal ECG was associated with more than 3 fold increased odds of having a culprit lesion and a positive predictive value of 92% and this result can be explained by understanding the pathophysiology of the ischemic changes on ECG. When insufficient myocardial perfusion is limited to subendocardial layer as in NSTEMI, the affected myocytes may lose their ability to maintain the prolonged activation that causes the normal similarity in the spatial directions of the QRS complex and T wave. The resultant ischemic T waves are inverted from positive to negative in many of the ECG leads because the axis of the ischemic T waves is directed away from the involved region of the left ventricle (20). On the other hand, shifting of the ST segment baseline occurs when insufficient perfusion causes the myocardial-cell membranes to become abnormally permeable to the flow of ions. The resulting difference in electrical potential between injured and uninjured myocardium causes a constant flow of injury current. When the injury is limited to the subendocardial layer of the left ventricle, the resultant “subendocardial injury” shifts the axis of the ST segment generally away from that ventricle, but not specifically away from the involved region. When insufficient perfusion extends through all of the myocardial layers (transmurally), the resultant epicardial ischemia shifts the T-wave axis toward the involved region of the left ventricle (20).
We recognize several limitations of the study. This was a retrospective study from a single tertiary referral center, with likely selection and referral bias. Furthermore, the sample size was relatively small and without long-term follow-up outcomes. In addition, patients were stratified based on the admission ECG; however, we do know that ECG changes are dynamic and we might have missed those changes by the time the patient presented to the emergency room. Also, in several cases, we did not know if the changes were new or old given that baseline ECG were not available for comparison to many patients. However, the ECGs were interpreted blindly without knowledge of the angiogram results to minimize any further bias.
Conclusions
In this single center retrospective database of patients admitted with NSTEMI and undergoing coronary angiography, abnormal admission ECG was an independent predictor of the presence of a culprit lesion with a high positive predictive value. Patient with normal admission ECG were more likely to have normal coronary arteries or LCX artery involvement when disease was identified.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. European Coronary Surgery Study Group. Lancet 1982;2:1173-80. [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 2013;127:e6-e245. [PubMed]
- Peterson ED, Roe MT, Mulgund J, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA 2006;295:1912-20. [PubMed]
- Fitzgerald P, Goodacre SW, Cross E, Dixon S. Cost-effectiveness of point-of-care biomarker assessment for suspected myocardial infarction: the randomized assessment of treatment using panel Assay of cardiac markers (RATPAC) trial. Acad Emerg Med 2011;18:488-95. [PubMed]
- Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation 2002;105:1760-3. [PubMed]
- Huey BL, Beller GA, Kaiser DL, et al. A comprehensive analysis of myocardial infarction due to left circumflex artery occlusion: comparison with infarction due to right coronary artery and left anterior descending artery occlusion. J Am Coll Cardiol 1988;12:1156-66. [PubMed]
- Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000;284:835-42. [PubMed]
- Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). Circulation 2000;102:1193-209. [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107-33. [PubMed]
- Raff GL, Abidov A, Achenbach S, et al. Scct guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr 2009;3:122-36. [PubMed]
- Matetzky S, Freimark D, Feinberg MS, et al. Acute myocardial infarction with isolated ST-segment elevation in posterior chest leads V7-9: "hidden" ST-segment elevations revealing acute posterior infarction. J Am Coll Cardiol 1999;34:748-53. [PubMed]
- Boden WE, Kleiger RE, Gibson RS, et al. Electrocardiographic evolution of posterior acute myocardial infarction: importance of early precordial ST-segment depression. Am J Cardiol 1987;59:782-7. [PubMed]
- Zalenski RJ, Rydman RJ, Sloan EP, et al. Value of posterior and right ventricular leads in comparison to the standard 12-lead electrocardiogram in evaluation of ST-segment elevation in suspected acute myocardial infarction. Am J Cardiol 1997;79:1579-85. [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2354-94. [PubMed]
- Melendez LJ, Jones DT, Salcedo JR. Usefulness of three additional electrocardiographic chest leads (V7, V8, and V9) in the diagnosis of acute myocardial infarction. Can Med Assoc J 1978;119:745-8. [PubMed]
- Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 2006;48:1475-97. [PubMed]
- ASCI CCT and CMR Guideline Working Group, Kitagawa K, Choi BW, et al. ASCI 2010 appropriateness criteria for cardiac magnetic resonance imaging: a report of the Asian Society of Cardiovascular Imaging cardiac computed tomography and cardiac magnetic resonance imaging guideline working group. Int J Cardiovasc Imaging 2010;26:173-86. [PubMed]
- Beckwith JR. Gant's clinical electrocardiography, 2nd ed. New York: McGraw-Hill, 1970:87-111.