Abrupt progression of ventricular septal perforation after primary angioplasty for acute myocardial infarction
Introduction
Although the occurrence of mechanical complication after acute myocardial infarction (MI) has decreased with the introduction of the primary coronary angioplasty, ventricular septal perforation (VSP) is still the leading cause of in-hospital mortality (1,2). Despite the recommendation of aggressive early surgical repair before the onset of hemodynamic deterioration for patients with VSP, mortality remains extremely high (3-7). Herein, we report an acute inferior MI showing immediate hemodynamic instability caused by progressive enlargement of VSP that was observed in serial echocardiography and was treated successfully through emergent surgical conversion.
Case report
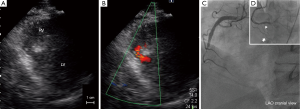
A 61-year-old man with a history of diabetes and chronic kidney disease was referred to his local hospital with chest discomfort and dyspnea. His symptoms had started 18 hours earlier. ECG showed ST segment elevation and abnormal Q waves in the inferior leads. Urgent transthoracic echocardiography revealed akinesis at the inferior wall with small VSP measuring 6 mm of the maximal diameter (Figure 1A,B). At this time, elevated creatine phosphokinase of 750 IU/L and serum creatinine of 3.0 mg/dL were observed. The patient underwent emergent coronary angiography, which revealed total occlusion of the mid-portion of the right coronary artery without collateral vessels (Figure 1C). Primary angioplasty was indicated for the occluded lesion due to persistence of the patient’s symptoms and ST-T changes. Thrombus aspiration and zotarolimus-eluting stent implantation led to partial reperfusion. Importantly, flow of the right ventricular branch and acute marginal branch were preserved after stenting (Figure 1D).
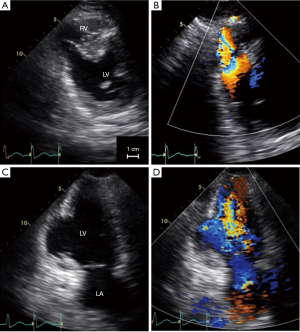
However, half an hour after the interventional procedure, hemodynamic instability occurred. The patient was then transferred to our hospital with an intra-aortic balloon pump in place. At that time, transthoracic echocardiography which was undertaken 3 hours after primary angioplasty, demonstrated progressive enlargement of the VSP (Figure 2). The ratio of pulmonary to systemic flow was 2.8. Thus, emergent surgical repair was indicated. While waiting for surgery, additional mechanical support using percutaneous cardiopulmonary support was necessary to maintain hemodynamics. Intraoperative transesophageal echocardiography, which was performed 6 hours after primary angioplasty, showed further development of the ventricular defect (Figures 3,4).


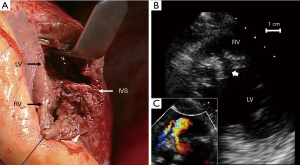
During operation, the large rupture site (Figure 5A) was repaired using the patch exclusion technique, as described by David et al. (10). Concomitant coronary artery bypass grafting (CABG) was not applied to the right coronary artery. Although there remained small residual VSP (Figure 5B,C), he was discharged on the 86th day after admission without complication except hemodialysis.
Discussion
Mechanical complications are relatively uncommon after acute MI. A previous study has shown that VSP with inferior infarction tended to have a worse outcome compared with anterior infarcts, due to the difficulty of surgical approach and the more frequent association with right ventricular infarction (7). One possible explanation for the successful recovery in this case is that the patient did not suffer from right ventricular infarction, which is compatible with preservation of the right ventricular branch flow that was detected with angiography.
The timing of surgery in patients with VSP remains controversial. This patient initially presented in a stable hemodynamic state that motivated the decision to not repair the VSP emergently; thus, primary angioplasty was performed for the recent inferior MI. After angioplasty, significant hemodynamic alterations occurred and urgent VSP repair was considered in addition to mechanical support. One of the largest studies of VSP cases showed that early repair was an independent predictor of 30-day mortality; however, this should not be interpreted as indicating that surgery should be delayed to reduce mortality (5). Delay of operation could allow the fragile myocardium to transform into firm tissue. On the other hand, the beneficial effects of a delay for a surgical indication are offset by the increases risk of multiple organ failure. Nishida et al. reported that preoperative percutaneous cardiopulmonary support was associated with in-hospital death (3). Under these conditions, the use of transcatheter closure device and stent graft to treat VSP complicating acute MI can be considered as an alternative therapy to open heart surgery (11-13).
As reported in the series of Takahashi et al. (6), concomitant CABG and preoperative acute percutaneous coronary intervention accounted for 63.5% and 13.5% of such cases, respectively. However, it remains controversial whether concomitant coronary revascularization improves outcome in patients with VSP. In this case, hemodynamic instability occurred suddenly in the catheterization laboratory while the patient was undergoing angioplasty for the culprit lesion. Cardiac magnetic resonance imaging suggested that late reperfusion appeared to increase the infarction area compared with conservative therapy for patients with ST-elevation MI (14). Among these patients, the supply of late coronary flow into the infarction area might influence the microvascular blood flow and induce reperfusion injury, thus resulting in enlargement of the infarction area. Even in the previous large-scale clinical investigators, it was hard to prove the clinical efficacy of percutaneous coronary intervention for patients with recent MI (15,16).
From this case, we suggest primary angioplasty may potentially induce late reperfusion injury in patients with VSP complicating MI.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nakatani D, Sato H, Kinjo K, et al. Effect of successful late reperfusion by primary coronary angioplasty on mechanical complications of acute myocardial infarction. Am J Cardiol 2003;92:785-8. [PubMed]
- Takii T, Yasuda S, Takahashi J, et al. Trends in acute myocardial infarction incidence and mortality over 30 years in Japan: report from the MIYAGI-AMI Registry Study. Circ J 2010;74:93-100. [PubMed]
- Nishida T, Sakakura K, Wada H, et al. Determinants of in-hospital death in patients with postinfarction ventricular septal perforation. Heart Vessels 2012;27:475-9. [PubMed]
- Morillon-Lutun S, Maucort-Boulch D, Mewton N, et al. Therapeutic management changes and mortality rates over 30 years in ventricular septal rupture complicating acute myocardial infarction. Am J Cardiol 2013;112:1273-8. [PubMed]
- Jeppsson A, Liden H, Johnsson P, et al. Surgical repair of post infarction ventricular septal defects: a national experience. Eur J Cardiothorac Surg 2005;27:216-21. [PubMed]
- Takahashi H, Arif R, Almashhoor A, et al. Long-term results after surgical treatment of postinfarction ventricular septal rupture. Eur J Cardiothorac Surg 2015;47:720-4. [PubMed]
- Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation 2000;101:27-32. [PubMed]
- Nakahashi T, Sakata K, Tsuda T, et al. Intraoperative transesophageal echocardiography from the four-chamber view showed further enlargement of the VSP. Asvide 2015;2:137. Available online: http://www.asvide.com/articles/713
- Nakahashi T, Sakata K, Tsuda T, et al. Intraoperative transesophageal echocardiography from the four-chamber view in color flow imaging. Asvide 2015;2:138. Available online: http://www.asvide.com/articles/714
- David TE, Dale L, Sun Z. Postinfarction ventricular septal rupture: repair by endocardial patch with infarct exclusion. J Thorac Cardiovasc Surg 1995;110:1315-22. [PubMed]
- Thiele H, Kaulfersch C, Daehnert I, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J 2009;30:81-8. [PubMed]
- Zhu XY, Qin YW, Han YL, et al. Long-term efficacy of transcatheter closure of ventricular septal defect in combination with percutaneous coronary intervention in patients with ventricular septal defect complicating acute myocardial infarction: a multicentre study. EuroIntervention 2013;8:1270-6. [PubMed]
- Watanabe G, Ohtake H, Tomita S, et al. Stent in the heart. J Am Coll Cardiol 2012;59:627. [PubMed]
- Khan JN, Razvi N, Nazir SA, et al. Prevalence and extent of infarct and microvascular obstruction following different reperfusion therapies in ST-elevation myocardial infarction. J Cardiovasc Magn Reson 2014;16:38. [PubMed]
- Hochman JS, Lamas GA, Buller CE, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006;355:2395-407. [PubMed]
- Dzavík V, Buller CE, Lamas GA, et al. Randomized trial of percutaneous coronary intervention for subacute infarct-related coronary artery occlusion to achieve long-term patency and improve ventricular function: the Total Occlusion Study of Canada (TOSCA)-2 trial. Circulation 2006;114:2449-57. [PubMed]


