Inflammation, plaque progression and vulnerability: evidence from intravascular ultrasound imaging
Introduction
A substantial body of evidence indicates that inflammatory pathway activation is important in the initiation and progression of atherosclerosis. The inflammatory cascade has been implicated during all stages of atheroma evolution, from the early development of endothelial dysfunction, through to formation of the mature atheroma and its subsequent rupture or erosion (1). These findings are supported by observations that elevated circulating biomarkers of inflammation, independently predict the likelihood of adverse cardiovascular outcomes in patients across a broad range of risk (2,3). As a result, there is an ongoing search to elucidate the factors that coordinate this complex cascade. The therapeutic approach specifically targeting inflammatory mediators involved in atherosclerosis could have significant clinical benefit.
Recent advancement in arterial wall imaging has expanded our ability to visualize the full extent of atheroma. Intravascular ultrasound (IVUS) enables high-resolution imaging of the arterial wall within the coronary vasculature. Increasing use of IVUS in clinical practice has enabled not only a more precise evaluation of percutaneous coronary intervention but also the serial evaluation of factors implicated in coronary atheroma progression and the efficacy of novel therapies on atherosclerotic plaques (Table 1). Other IVUS systems such as radiofrequency backscatter IVUS enables plaque composition analysis (Table 1). These modalities have provided important information with regard to the natural history of atherosclerosis, factors influencing plaque changes and the efficacy of anti-atherosclerotic medical therapies. IVUS has also helped elucidate the contribution of inflammation to atherosclerosis in vivo. The present review provides evidences illustrating the relationship of inflammation with atherosclerotic plaque.
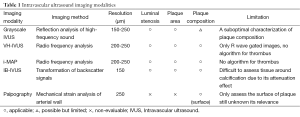
Full table
Inflammatory mediators and atherosclerotic plaques
IVUS imaging studies have reported the effect of inflammatory mediators on atherosclerotic plaques (Table 2).
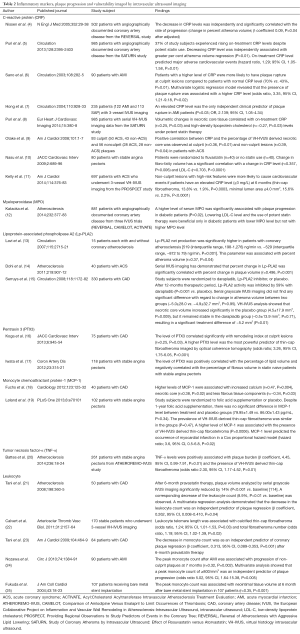
Full table
C-reactive protein (CRP)
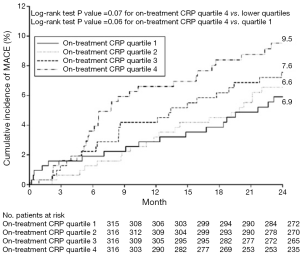
CRP is an acute-phase reactant and nonspecific marker of inflammation, produced predominantly in hepatocytes as a pentamer of identical subunits in response to several cytokines. Recent imaging studies using gray scale IVUS have elucidated the relationship between CRP and plaque progression. The REVERSAL (reversal of atherosclerosis with aggressive lipid lowering) study has demonstrated less plaque progression was observed in patients who achieved lower low-density lipoprotein cholesterol (LDL-C) level with atorvastatin [change in percent atheroma volume; 0.2% (95% CI, −0.3 to 0.5) vs. 1.6% (95% CI, 1.2-2.2), P<0.001] (4). Further analysis has elucidated the decrease in CRP levels was independently and significantly correlated with the rate of progression (change in percent atheroma volume; β coefficient 0.09, P=0.04 after adjusted) (4). The SATURN (study of coronary atheroma by IVUS: effect if rosuvastatin versus atorvastatin) trial compared the effects of 80 mg atorvastatin and 40 mg rosuvastatin on plaque progression (26). In this study, both therapeutic regimens achieved very low LDL-C levels (70.2±1.0 vs. 62.6±1.0 mg/dL, P<0.001) and were associated with marked regression of coronary atherosclerosis [change in percent atheroma volume; −0.99 (95% CI, −1.19 to 0.63) vs. −1.22 (95% CI, −1.52 to −0.90, P=0.17)]. A post-hoc analysis of SATURN investigated the impact of CRP control on plaque progression and cardiovascular outcomes under maximally intensive statin therapy (5). Despite achieving very low LDL-C level under potent statin therapy, 37% of study subjects experienced rising on-treatment CRP levels. Decreasing CRP level was independently associated with greater percent atheroma volume regression (P=0.01). In addition, on-treatment CRP level was independently associated with major adverse cardiovascular events (hazard ratio, 1.29; 95% CI, 1.05-1.58, P=0.01) (Figure 1). These findings suggest that systemic inflammation reflected by CRP level may contribute to ongoing cardiovascular risk in patients receiving high-intensity statin therapy.
Recently, virtual histology-IVUS (VH-IVUS) has been used to analyze the relationship of CRP with plaque composition. VH-IVUS imaging analysis in SATURN has demonstrated that volumetric changes in necrotic core tissue correlated with on-treatment CRP (r=0.25, P=0.03) and high-density lipoprotein cholesterol (r=−0.27, P=0.03) levels during potent statin therapy (8). The PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) study investigated the predictive ability of VH-IVUS derived plaque features for cardiovascular events in patients with acute coronary syndrome (ACS) (27). The sub-analysis of the PROSPECT study revealed that non-culprit lesions were more likely to cause cardiovascular events if patients have an elevated CRP level (≥3 mg/L) at 6 months (thin-cap fibroatheroma, 13.8% vs. 1.9%, P=0.0003; minimal lumen area ≤4.0 mm2; 15.6% vs. 2.2%, P<0.0001) (11). As such, grayscale and VH-IVUS imaging studies indicate a critical role of CRP in identifying the presence of plaque progression and vulnerability.
Myeloperoxidase (MPO)
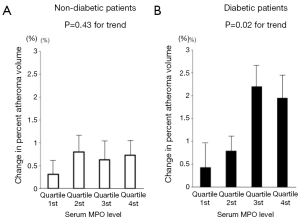
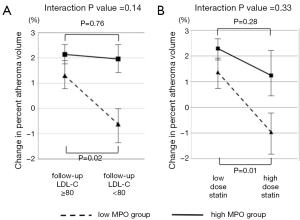
MPO is a pro-oxidant enzyme released from granules of activated neutrophils, monocytes, and certain tissue macrophages (28). MPO promotes oxidation of low-density lipoprotein, and apolipoprotein A-I in high-density lipoproteins, imparing its ability to promote reverse cholesterol transport. This combination of detrimental effects suggests MPO as an active mediator of atherogenesis. We recently reported the relationship between serum MPO level and plaque progression in 881 patients with coronary artery disease (CAD) (12). There was no relationship between MPO level and plaque progression in non-diabetic patients (P=0.43). In contrast, a higher level of serum MPO was significantly associated with plaque progression in diabetic patients (P=0.02) (Figure 2). Interestingly, lowering LDL-C levels and the use of potent statin therapy were beneficial only in diabetic patients with lower MPO level but not with higher MPO level (Figure 3). These findings may indicate the importance of MPO pathways in diabetic cardiovascular disease. Modifying systemic MPO activity within diabetic patients may be a viable pharmacologic strategy, and a targeted reduction of MPO activity, particularly within diabetics, may become feasible in the future.
Lipoprotein-associated phospholipase A2 (Lp-PLA2)
Lp-PLA2 is an enzyme belonging to the A2 phospholipase superfamily, which associates with atherosclerosis. Gray scale IVUS imaging studies identified the relationship between Lp-PLA2 and coronary atherosclerosis. In one study, authors conducted IVUS imaging and collected plasma samples from the left main coronary artery and coronary sinus to measure net production of Lp-PLA2 in 15 patients with and without coronary atherosclerosis (13). Lp-PLA2 net production was significantly higher in patients with coronary atherosclerosis (P=0.001). In addition, this parameter was significantly associated with percent atheroma volume (r=0.37, P=0.04).
Observational data showing the contribution of Lp-PLA2 to atherosclerosis has emerged, supporting the concept that Lp-PLA2 inhibition might favourably modulate atherosclerotic plaques, potentially leading to the prevention of cardiovascular events. Serruys et al. investigated the efficacy of the direct Lp-PLA2 inhibitor, darapladib on coronary atherosclerotic plaque in 330 patients with CAD (15). After a 12-months therapeutic period, Lp-PLA2 activity was inhibited by 59% with darapladib (P<0.001 vs. placebo). Serial gray scale IVUS imaging did not find any significant difference with regard to change in atheroma volume (−5.0±28.0 vs. −4.9±32.7 mm3, P=0.95). However, VH-IVUS analysis showed that necrotic core volume increased significantly in the placebo group (4.5±17.9 mm3, P=0.009), remaining stable in the darapladib group (−0.5±13.9 mm3, P=0.71), resulting in a significant treatment difference of −5.2 mm3 (P=0.01). This finding suggested the potential of darapladib to reduce cardiovascular events by stabilizing plaques. However, a recent large-scale prospective double-blind randomized trial failed to demonstrate a reduction in major adverse cardiovascular events in patients with stable CAD disease (hazard ratio in the darapladib group, 0.94; 95% CI, 0.85-1.03; P=0.20) (29).
Pentraxin 3 (PTX3)
PTX3 is a member of the pentraxin superfamily, which are multifunctional pattern-recognition proteins characterized by a cyclic multimeric structure. Immunohistochemical staining of advanced atherosclerotic lesions has revealed a strong expression of PTX3 on the lumen surface as well as within the atherosclerotic plaque in animal models and in humans (30).
Recent imaging studies described the association between PTX3 and atherosclerotic plaques in patients with CAD. Koga et al. , reported that the level of PTX3 correlated significantly with the remodeling index of culprit lesions in 75 patients with CAD (r=0.25, P=0.03) (16). In addition, a higher PTX3 level was the most powerful predictor of thin-cap fibroatheroma imaged by optical coherence tomography (odds ratio, 3.26; 95% CI, 1.75-6.05, P<0.001). Another study using IB-IVUS imaging demonstrated that the level of PTX3 was positively correlated with the percentage of lipid volume and negatively correlated with the percentage of fibrous volume in statin naïve patients with stable angina pectoris (17). These observations suggest the important role of PTX3 in atherosclerosis and cardiovascular outcomes.
Monocyte chemoattractant protein-1 (MCP-1)
MCP-1, the first and prototypic chemokine, induced the intensive infiltration of macrophages into plaques with active inflammation. In one imaging study using VH-IVUS (18), higher levels of MCP-1 were associated with increased calcium (r=0.47, P=0.004), necrotic core (r=0.38, P=0.02) but less fibrous tissue components (r=−0.34, P=0.03). Based on findings showing a reduction of MCP-1 under folic acid supplementation, a randomized, prospective study was designed to evaluate the effect of folic acid supplementation on plaque vulnerability in 102 patients with stable angina pectoris (19). Despite 1-year folic acid supplementation schedule, there was no significant difference in MCP-1 levels between treatment and placebo groups (79.95±1.49 vs. 86.00±1.43 pg/mL, P=0.34). Moreover, the prevalence of VH-IVUS derived thin-cap fibroatheroma was similar in the groups (P=0.47). However, a post-hoc analysis identified a higher level of MCP-1 to associate with the presence of VH-IVUS derived thin-cap fibroatheroma (P=0.0006). Also, MCP-1 levels predicted the occurrence of myocardial infarction in a Cox proportional hazard model (P=0.02).
Tumor necrosis factor-α (TNF-α)
TNF-α is a pro-inflammatory cytokine linked to a higher incidence of a variety of diseases including coronary atherosclerosis. The ATHEROREMO-IVUS (the European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis-Intravascular Ultrasound) study sought to investigate the association of atherogenic cytokines with the extent and composition of coronary atherosclerosis by using gray scale and VH-IVUS (20). In 261 patients with stable angina pectoris, TNF-α levels were positively associated with plaque burden (β coefficient, 4.45; 95% CI, 0.99-7.91, P=0.01) and the presence of VH-IVUS derived thin-cap fibroatheroma (odds ratio, 2.30; 95% CI, 1.17-4.52, P=0.01).
Leukocyte count
Tani et al. evaluated the association between circulating leukocyte count with coronary atherosclerosis in 50 patients treated with pravastatin. After 6-month therapy, plaque volume analyzed by serial gray scale IVUS imaging significantly reduced by 14% (P<0.001 vs. baseline) (23). A corresponding decrease of the leukocyte count (8.9%, P<0.01 vs. baseline) was observed. Multivariate regression analysis demonstrated that the decrease in the leukocyte count independently predicted plaque regression (β coefficient, 0.26; 95% CI, 0.01-0.41, P=0.04). Another study investigated the effect of leukocyte telomere length, a marker of leukocyte senescence on plaque phenotype (22). In 170 stable patients who underwent 3-vessel VH-IVUS imaging, leukocyte telomere length was associated with calcified thin-cap fibroatheroma (odds ratio, 1.24; 95% CI, 1.01-1.53, P=0.03) and total fibroatheroma number (odds ratio, 1.19; 95% CI, 1.02-1.39, P=0.02).
Inflammation, vulnerable plaques and atheroma progression
Inflammation has been reported to play a key role in promoting plaque vulnerability via thinning fibrous caps, enhancing the influx of lipids and expanding the lipid core, and stimulating neoangiogenesis (31). Patho-histological studies have characterized vulnerable plaques as a presence of large lipid core, thin fibrous cap, macrophage infiltration, spotty calcification and neovascularization (32). Of these features, spotty calcification has been demonstrated to represent a dynamic process associated with inflammatory activity (33). Serial gray scale IVUS imaging elucidated that spotty calcification was associated with an accelerated plaque progression, reflected by a greater change in percent atheroma volume (0.68%±0.12% vs. 0.05%±0.17%, P=0.002) (34).
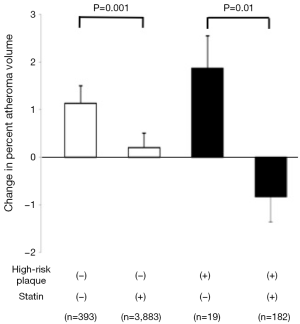
Based on patho-histological findings about the morphology of vulnerable plaques, we defined gray scale IVUS derived high-risk plaque as a presence of spotty calcification, large plaque burden and positive remodeling, and evaluated this with regard to plaque progression in 4,477 stable CAD patients (35). In this analysis, substantial plaque progression was observed in patients having high-risk plaques who did not receive a statin (change in percent atheroma volume: 1.87±0.68). However, these subjects exhibited plaque regression under a statin (change in percent atheroma volume: −0.83%±0.53%) (Figure 4). These observations might also support the association of inflamed burden with its progression. Given that the rate of progression in serial observations were associated with a greater likelihood of death, myocardial infarction and coronary revascularization (36), these findings may suggest the need to modify atherosclerotic plaques containing vulnerable features associated with inflammation to prevent future cardiovascular events.
Another inflamed high-risk plaque, the thin-cap fibroatheroma has been investigated in recent studies using VH-IVUS. The PROSPECT study conducted 3-vessel VH-IVUS imaging in 697 patients with ACS and evaluated the relationship between plaque features and major adverse cardiovascular events during 3-year follow up (27). Non-culprit lesions causing events were more likely to exhibit a larger plaque burden ≥70% (hazard ratio, 5.03; 95% CI, 2.51-10.11, P<0.001), a minimal luminal area <4.0 mm2 (hazard ratio, 3.21; 95% CI, 1.61-6.42, P=0.001), or VH-IVUS derived thin-cap fibroatheroma (hazard ratio, 3.35; 95% CI, 1.77-6.36, P<0.001). The VIVA (VH-IVUS in Vulnerable Atherosclerosis) study also reported similar findings showing the relationship of VH-IVUS derived TCFA (hazard ratio, 8.16; 95% CI, 1.78-37.32, P=0.007), plaque burden >70% (hazard ratio, 7.48; 95% CI, 2.50-22.31, P<0.001) and minimum luminal area <4.0 mm2 (hazard ratio, 2.91; 95% CI, 1.07-7.91, P=0.03) with major adverse cardiovascular events (37).
Summary
Recent imaging studies have affirmed the contribution of inflammation to plaque development, progression and vulnerability. Also, the presence of plaques with inflammatory components associates with a greater likelihood of future cardiovascular events. These observations outline the potential benefits of therapies targeting inflammation in the arterial wall. With ongoing technical advances, IVUS imaging will continue to play a critical role in the evaluation of novel compounds designed to modulate inflammation.
Acknowledgements
None.
Footnote
Conflicts of Interest: Y Kataoka has received research support from Cerenis. SJ Nicholls has received speaking honoraria from AstraZeneca, Pfizer, Merck Schering-Plough and Takeda, consulting fees from AstraZeneca, Pfizer, Merck Schering-Plough, Takeda, Roche, NovoNordisk, LipoScience and Anthera and research support from AstraZeneca and Lipid Sciences.
References
- Libby P. . Inflammation in atherosclerosis. Nature 2002;420:868-74. [PubMed]
- Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation 2004;109:1955-9. [PubMed]
- Haverkate F, Thompson SG, Pyke SD, et al. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet 1997;349:462-6. [PubMed]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med 2005;352:29-38. [PubMed]
- Puri R, Nissen SE, Libby P, et al. C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation 2013;128:2395-403. [PubMed]
- Sano T, Tanaka A, Namba M, et al. C-reactive protein and lesion morphology in patients with acute myocardial infarction. Circulation 2003;108:282-5. [PubMed]
- Hong MK, Mintz GS, Lee CW, et al. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation 2004;110:928-33. [PubMed]
- Puri R, Libby P, Nissen SE, et al. Long-term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging 2014;15:380-8. [PubMed]
- Otake H, Shite J, Shinke T, et al. Relation between plasma adiponectin, high-sensitivity C-reactive protein, and coronary plaque components in patients with acute coronary syndrome. Am J Cardiol 2008;101:1-7. [PubMed]
- Nasu K, Tsuchikane E, Katoh O, et al. Effect of fluvastatin on progression of coronary atherosclerotic plaque evaluated by virtual histology intravascular ultrasound. JACC Cardiovasc Interv 2009;2:689-96. [PubMed]
- Kelly CR, Weisz G, Maehara A, et al. Relation of C-reactive protein levels to instability of untreated vulnerable coronary plaques (from the PROSPECT Study). Am J Cardiol 2014;114:376-83. [PubMed]
- Kataoka Y, Shao M, Wolski K, et al. Myeloperoxidase levels predict accelerated progression of coronary atherosclerosis in diabetic patients: insights from intravascular ultrasound. Atherosclerosis 2014;232:377-83. [PubMed]
- Lavi S, McConnell JP, Rihal CS, et al. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation 2007;115:2715-21. [PubMed]
- Dohi T, Miyauchi K, Okazaki S, et al. Decreased circulating lipoprotein-associated phospholipase A2 levels are associated with coronary plaque regression in patients with acute coronary syndrome. Atherosclerosis 2011;219:907-12. [PubMed]
- Serruys PW, García-García HM, Buszman P, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 2008;118:1172-82. [PubMed]
- Koga S, Ikeda S, Yoshida T, et al. Elevated levels of systemic pentraxin 3 are associated with thin-cap fibroatheroma in coronary culprit lesions: assessment by optical coherence tomography and intravascular ultrasound. JACC Cardiovasc Interv 2013;6:945-54. [PubMed]
- Iwata A, Miura S, Tanaka T, et al. Plasma pentraxin-3 levels are associated with coronary plaque vulnerability and are decreased by statin. Coron Artery Dis 2012;23:315-21. [PubMed]
- Fuchs S, Lavi I, Tzang O, et al. Intracoronary monocyte chemoattractant protein 1 and vascular endothelial growth factor levels are associated with necrotic core, calcium and fibrous tissue atherosclerotic plaque components: an intracoronary ultrasound radiofrequency study. Cardiology 2012;123:125-32. [PubMed]
- Løland KH, Bleie Ø, Strand E, et al. Effect of folic acid supplementation on levels of circulating Monocyte Chemoattractant Protein-1 and the presence of intravascular ultrasound derived virtual histology thin-cap fibroatheromas in patients with stable angina pectoris. PLoS One 2013;8:e70101. [PubMed]
- Battes LC, Cheng JM, Oemrawsingh RM, et al. Circulating cytokines in relation to the extent and composition of coronary atherosclerosis: results from the ATHEROREMO-IVUS study. Atherosclerosis 2014;236:18-24. [PubMed]
- Tani S, Nagao K, Anazawa T, et al. Association of circulating leukocyte count with coronary atherosclerosis regression after pravastatin treatment. Atherosclerosis 2008;198:360-5. [PubMed]
- Calvert PA, Liew TV, Gorenne I, et al. Leukocyte telomere length is associated with high-risk plaques on virtual histology intravascular ultrasound and increased proinflammatory activity. Arterioscler Thromb Vasc Biol 2011;31:2157-64. [PubMed]
- Tani S, Nagao K, Anazawa T, et al. Association of leukocyte subtype counts with coronary atherosclerotic regression following pravastatin treatment. Am J Cardiol 2009;104:464-9. [PubMed]
- Nozawa N, Hibi K, Endo M, et al. Association between circulating monocytes and coronary plaque progression in patients with acute myocardial infarction. Circ J 2010;74:1384-91. [PubMed]
- Fukuda D, Shimada K, Tanaka A, et al. Circulating monocytes and in-stent neointima after coronary stent implantation. J Am Coll Cardiol 2004;43:18-23. [PubMed]
- Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 2011;365:2078-87. [PubMed]
- Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226-35. [PubMed]
- Nicholls SJ, Hazen SL. Myeloperoxidase, modified lipoproteins, and atherogenesis. J Lipid Res 2009;50:S346-51. [PubMed]
- STABILITY Investigators. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med 2014;370:1702-11. [PubMed]
- Rolph MS, Zimmer S, Bottazzi B, et al. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2002;22:e10-4. [PubMed]
- Bentzon JF, Otsuka F, Virmani R, et al. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852-66. [PubMed]
- Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262-75. [PubMed]
- New SE, Goettsch C, Aikawa M, et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res 2013;113:72-7. [PubMed]
- Kataoka Y, Wolski K, Uno K, et al. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J Am Coll Cardiol 2012;59:1592-7. [PubMed]
- Kataoka Y, Wolski K, Balog C, et al. Progression of coronary atherosclerosis in stable patients with ultrasonic features of high-risk plaques. Eur Heart J Cardiovasc Imaging 2014;15:1035-41. [PubMed]
- Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399-407. [PubMed]
- Calvert PA, Obaid DR, O'Sullivan M, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovasc Imaging 2011;4:894-901. [PubMed]


