Intravascular imaging in Kounis syndrome: role of IVUS and OCT in achieving an etiopathogenic diagnosis
Very late stent thrombosis (VLST) is a serious complication, which mainly affects drug-eluting stents (DES) and it is produced by multiple mechanisms. We present a rare case of VLST that occurred in a stent fracture site during an anaphylactic shock. In this report, intravascular imaging techniques were used, which were decisive to know the pathogenesis of this VLST triggered by a severe allergic response.
A 60-year-old man presented to the emergency room with sudden onset of generalized erythema, pruritus and progressive dyspnea three hours after the ingestion of uncooked anchovies. His past medical history included type 2 diabetes mellitus, hypercholesterolemia, hypertension and ischemic heart disease, with a NSTEMI 6 years ago that required implantation of a DES (Cypher 3.5 mm × 24 mm) in the mid right coronary artery. Two years later, a new coronary angiography was performed due to an electrically equivocal EKG stress test, demonstrating no restenosis signs of the previous coronary intervention. However, a stent fracture was evidenced in the middle of the stent (Figure 1A) but no further therapy was conducted given the patient’s excellent clinical status and the vessel patency (Figure 1B).
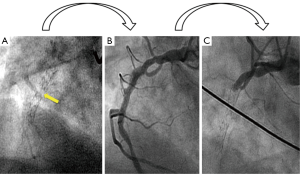
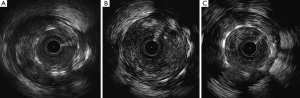
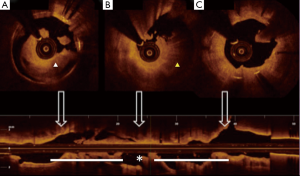
Soon after his admission, the patient presented malaise with nausea and hypotension (BP: 70/40 mmHg). Due to suspected anaphylactic shock, 0.3 mg of intramuscular epinephrine and 200 mg intravenous hydrocortisone were administered. Immediately after, the patient reported stabbing chest pain, and the ECG showed persistent ST segment elevation in inferior leads. An emergent coronary angiography was performed within 30 minutes of onset of symptoms and evidenced a VLST of the previous implanted stent (Figure 1C). After crossing the stent thrombosis with a guide wire, manual thrombus aspiration was performed, obtaining a macroscopically white thrombus and two small samples (1 mm × 1 mm) of red thrombus. Despite subsequent thrombus aspirations and low-pressure inflation with 2 mm × 10 mm semi-compliant balloon, a severe angiographic depletion defect persisted, mainly confined to the fracture region and 15 mm distally. Since the depletion defect persisted despite the previous maneuvers and in order to study the etiopathogenic diagnosis of this VLST, an intracoronary IVUS was conducted, demonstrating in addition to the stent fracture a positive remodeling of the artery with adequate apposition and expansion of the stent (Figure 2). However, at the site of stent fracture and distally there was a dense and homogeneous material, which IVUS was not able to differentiate between thrombus and late restenosis or neoatherosclerosis. An OCT was therefore performed, clearly demonstrating an irregular intraluminal mass, signal-rich but without light attenuation, attached to the wall in the stent fracture site and freely floating distally. All this was compatible with the presence of a large amount of white thrombus, attached to the fracture site (Figure 3). The optical characteristics of the mass (very low light attenuation) also allowed OCT to better analyze the vessel wall characteristics distal to the thrombus, demonstrating the absence of restenosis or neoatherosclerosis, which IVUS was not be able to recognize. Similar to IVUS, a good apposition and expansion of the underlying stent was seen.
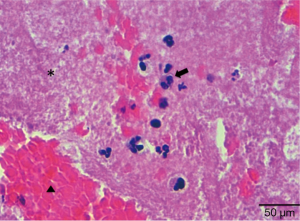
The procedure was concluded with the implantation of a zotarolimus DES (3.5 mm × 30 mm) in the thrombosed segment. Final IVUS and OCT showed good expansion and stent apposition. An echocardiogram demonstrated good ventricular function and the patient was discharged 3 days after intervention with double antiplatelet therapy (aspirin 100 mg/day and prasugrel 10 mg/day). The Allergology Department confirmed hypersensitivity to Anisakis Simplex with serologic tests. Pathological examination of the thrombotic material evidenced platelet aggregates, fibrin and white blood cells that included eosinophils. There were only scarce red cells (Figure 4). All of these features were compatible with a very early stage of white thrombus formation.
VLST that occurs more than one year after stent implantation, although infrequent, is a major concern in DES era since it is associated with large STEMI and sudden death. Even though its mechanisms are not completely understood, it is known that stent malapposition, uncovered stents, fractures, severe restenosis and neoatherosclerosis may play among others, a role in the pathogenesis of VLST related to DES (1). All VLST causes except stent fracture were better excluded by OCT than by IVUS. The incidence of stent fracture by angiography and IVUS has been reported to be 1-2%, although an incidence as high as 29% has been described in autopsy studies (2).
Stent fracture in our patient was evidenced in a previous coronary angiography performed four years before and did not have any clinical implications until the allergic reaction. Hence, we believe that in absence of other mayor causes of VLST as shown by OCT, it was the hypersensibility reaction what triggered the VLST, along with a favorable substrate as the stent fracture. Eosinophils and mast cells have been proven to be present in areas with stent fractures (3) and their degranulation following an allergic insult could induce platelet aggregation and subsequent thrombus formation. The occurrence of an acute coronary syndrome during the course of an allergic reaction is known as Kounis syndrome (3), and type III Kounis syndrome is defined as coronary artery stent thrombosis with presence of eosinophils and mast cells in the aspirated thrombus. The absence of a high number of eosinophils in the thrombotic material in our patient may be due to the very short period of time between the onset of symptoms and the aspiration, in which fibrin is formed in the presence of a small number of platelet aggregates. As a matter of fact, in most of the cases described in literature histological analysis was performed hours after the event or even during autopsies (4). OCT predicted that it would be a white thrombus in the histology as it was signal-rich and had low backscattering, contrary to red thrombi which have high backscattering and signal-free shadowing OCT characteristics (5).
We present for the first time a VLST in a stent fracture site induced by an allergic reaction (Kounis syndrome, type III) six years after a DES implantation. In addition, this is to the best of our knowledge the first report in the literature of a Kounis syndrome in which intracoronary imaging techniques (IVUS and OCT) have been used. They both localized the stent fracture, however OCT but not IVUS was key to clearly demonstrate the presence of intracoronary white thrombus, and to discard other known causes of VLST. For these reasons, we believe that OCT should be used as a first option in cases such as this type III Kounis syndrome, where a very early stage thrombus is expected to be found. Finally, this case could explain a new physiopathological mechanism of stent thrombosis occurring in stent fractures, since stent fracture sites contain eosinophils and mast cells and may be a favorable substrate for platelet aggregation and subsequent thrombus formation during a hypersensibility reaction.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kirtane AJ, Stone GW. How to minimize stent thrombosis. Circulation 2011;124:1283-7. [PubMed]
- Nakazawa G, Finn AV, Vorpahl M, et al. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol 2009;54:1924-31. [PubMed]
- Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther 2013;35:563-71. [PubMed]
- Chen JP, Hou D, Pendyala L, et al. Drug-eluting stent thrombosis: the Kounis hypersensitivity-associated acute coronary syndrome revisited. JACC Cardiovasc Interv 2009;2:583-93. [PubMed]
- Kang SJ, Lee CW, Song H, et al. OCT analysis in patients with very late stent thrombosis. JACC Cardiovasc Imaging 2013;6:695-703. [PubMed]


