The subcutaneous ICD—current evidence and challenges
Introduction
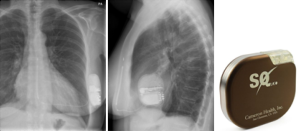
The subcutaneous implantable cardioverter-defibrillator (S-ICD, Figure 1) represents a paradigm shift in ICD technology. Its primary advantage of negating the risks associated with transvenous systems makes it an alluring alternative to the conventional ICD particularly in younger patients who are exposed to the risks of chronic intravascular lead complications. Whilst long-term data is currently unavailable, initial studies demonstrate that this is a promising and viable successor to transvenous devices, with comparable efficacy. However, the evidence that supports this has been questioned in the literature, and the controversies regarding certain technological and epidemiological aspects of the S-ICD warrant further evaluation.
Current evidence from EFFORTLESS and IDE
The EFFORTLESS study, a non-randomised, standard of care, multicentre registry, was the first of its kind to evaluate the clinical utility and performance efficacy of subcutaneous ICDs on an international scale. Consisting of a total population of 472 patients, 241 were enrolled prospectively and had a mean follow-up duration of 558 days (range, 13-1,342 days, median 498 days) with a mean age of 49±18 years (range, 9-88 years). A total of 317 spontaneous episodes were recorded in 95 patients during the follow-up period, of which 169 received therapy and 93 of these were for ventricular tachycardia (VT)/ventricular fibrillation (VF). With respect to discrete VT/VF episodes, first shock conversion efficacy was 88% with 100% overall successful clinical conversion after a maximum of five shocks. Overall, conversion efficacy of spontaneous episodes was 96.1% (95% CI: 90.8-100%). In the two instances in which shock therapy failed according to study-definition (delayed conversion outside EGM storage time and undersensing causing a new episode to be reported), successful conversion was achieved shortly after. In reality, therefore, there was a clinical 100% conversion efficacy as all discrete VT/VF were converted to sinus rhythm. Of the 39 episodes of non-sustained VT (NSVT)/VF, 37 were of a duration shorter than the initial detection duration and therefore did not initiate shock therapy. In the two remaining instances, the VT/VF rhythm self-terminated after detection but prior to shock delivery. Six VT/VF storm events in four patients resulted in the 40 episodes. In one case of a patient with Loeffler’s syndrome, the VF storm was preceded by a 10-minute period of bradycardia (lowest heart rate of 28/min in the 60 s pre-arrest). The VF that subsequently developed was not successfully defibrillated, and the patient died. This was an unusual case in which the patient had obliteration of the right and left ventricular apices by a mass and was not deemed suitable for a standard ICD system. At implant, VF had been sensed appropriately and cardioverted at 65 J. The 360-day inappropriate shock rate was 7% with T wave oversensing being the major cause (1).
These statistics are comparable to data from FDA Investigational Device Exemption (IDE) study, another prospective non-randomised multicentre trial that studied adults with ICD indications who did not require pacing or have documented pace-terminable VT (2). Comprising 304 patients, the study demonstrated almost 100% acute conversion, with a 95% lower confidence limit of 98.8%, which exceeded the pre-specified objective performance goal of ≥88%. A total of 119 spontaneous VT/VF episodes were treated by shock therapy, of which 38 were discrete VT/VF episodes and 81 VT/VF storms. The 38 discrete episodes, comprising of 22 monomorphic VT episodes and 16 of polymorphic VT/VF, received 43 appropriate shocks that successfully terminated the dysrhythmia. Thirty-five of the 38 episodes (92.1%) were converted on first shock and 37/38 (97.4%) with one or more shocks. The single unsuccessful episode of monomorphic VT terminated spontaneously while the device was charging to deliver a second shock, and a subsequent episode in the same patient was successfully terminated on first shock. A total of 75% of VT/VF storms were terminated successfully; one storm was terminated by externally administered shock therapy whilst the device was charging in an emergency situation. Importantly, no arrhythmic deaths were reported. Further still, the chronic conversion substudy demonstrated a 96% success rate at 65 J shock therapy, with the remaining successful at detecting and converting VF at 80 J (2). These results suggest that the S-ICD is effective in terminating arrhythmias in the acute and chronic setting, and moreover, they were corroborated by a German study which demonstrated equivalent first and second shock defibrillation efficacy between S-ICDs and transvenous ICDs (TV-ICD) (3). They also demonstrate the robust clinical efficacy of S-ICDs beyond the confines of defibrillator threshold (DFT) testing and in the context of real-life metabolic disturbance, that is not dissimilar to TV-ICDs and which supports the use of S-ICDs in the wider patient population.
Two vs. three incision technique for inserting S-ICD
The conventional S-ICD implantation technique involves electrode and device implantation by three incisions; one for the lateral pocket and two parasternal incisions. The electrode is tunnelled from the lateral pocket through the parasternal incisions before being sutured in situ. Of note, the superior parasternal incision is prone to exposure, and therefore susceptible to infection, more likely to cause discomfort and may be aesthetically less acceptable than the other incision sites. Knops et al. (4) have described a two-incision technique for S-ICD as a solution that avoids the third superior parasternal incision, and demonstrated similar if not better efficacy to the three-incision technique. Of the 39 patients who underwent the two-incision technique, and over more than 14 months follow-up, they report no lead dislocations or requirement to reposition it. There were only two superficial wound infections of the pocket site and no inappropriate sensing occurred relating to implantation technique. The likelihood of long-term lead migration is thought to be minimised as a result of fibrotic tissue forming around the electrode that would provide additional structural support. Furthermore, as the technique was trialed in a young and more active population, it is felt that these results can be reliably extrapolated to an older cohort of patients who are arguably at less risk of dislocation-related complications in the first instance.
Sensing ventricular arrhythmia
By virtue of its design, the S-ICD sensing system is different from the conventional ICD. Table 1 (5) highlights the main differences between S-ICDs and TV-ICDs. S-ICDs have two programmable zones of tachycardia detection: a supraventricular tachycardia (SVT) discrimination zone and a VF zone. Whilst the latter is purely dictated by ventricular rate, the former utilises a number of parameters including ECG morphology and stability to differentiate between VT/VF and SVT. Therapy is then withheld if SVT discrimination criteria are met below the VF therapy heart rate threshold. These algorithms may be ineffective if the patient develops bundle branch block during SVT, although this can be overcome by employing an ECG template recording aberrant beat morphology if it has been recognised during screening (6). In this manner, in addition to continuously monitoring the heart rate, the S-ICD also compares the QRS-complex and T-wave morphology to a template registered and stored by the S-ICD immediately after implantation to discriminate between SVT and ventricular arrhythmias. For this reason also, QRS-T wave morphology screening and analysis is integral to assessing device eligibility and efficacy prior to implantation. For example, following QRS complex and T wave morphology screening in 230 patients with ICDs who had no indications for cardiac pacing, Olde Nordkampe et al. (7) demonstrated that 7.4% would not have been eligible for a S-ICD. Independent predictors for screening failure were hypertrophic cardiomyopathy [odds ratio (OR), 12.6], a high BMI (OR, 1.5), prolonged QRS duration (OR, 1.5) and a R:T ratio <3 in the lead with the largest T wave on a standard 12-lead surface ECG (OR, 14.6). It is therefore clear that the S-ICD may be inappropriate for a small, albeit significant fraction of the population who would otherwise benefit from a conventional ICD. Nevertheless, the START study demonstrated equivalent sensitivity of S-ICDs and TV-ICDs in detecting VF, but superior specificity of S-ICDs to discriminate between arrhythmia compared to single and dual chamber TV-ICDs (8). Reassuringly, this is consistent with reports from the IDE study that demonstrated no inappropriate shocks as a result of misclassification of atrial fibrillation (AF) or SVT and low incidence of such events in EFFORTLESS (9).
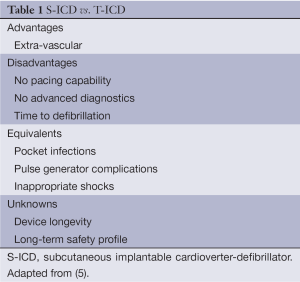
Full table
Minimising T wave oversensing
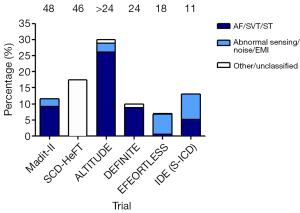
A persistent challenge for S-ICDs is their propensity to over-sense T-waves, in which the T-wave is incorrectly recognised as a QRS complex resulting in double counting, and subsequent delivery of inappropriate shocks (Figure 2). Randomised controlled trials have suggested that the incidence for this phenomenon is 12-17% (12,14-16). The IDE trial reported an inappropriate shock delivery in 13% of patients over an 11-month follow-up period (2). Over-sensing caused inappropriate shocks in 25 patients, of which 22 experienced over-sensing specifically of the T-wave. This was a significantly ameliorated with dual zone rather than single zone programming. Similarly in the EFFORTLESS study, most shocks were due to over-sensing of cardiac signals; only 4 patients had inappropriate shocks due to noise or EMI and 6 due to SVT (1).
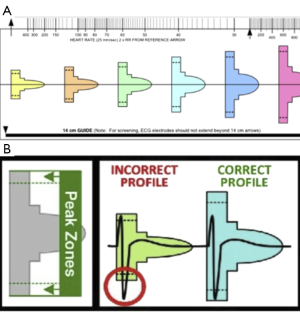
To address this matter, a screening template has been designed by S-ICD manufacturers to identify patients vulnerable to over-sensing prior to insertion (Figure 3) (17). Groh et al. (17) demonstrated that 8% of patients who already had an ICD and were not paced would fail the screening test, confirming their susceptibility to T-wave over-sensing. In particular, T-wave inversions (TWI) as seen in ischaemia and hypertrophy in leads I, II, and aVF on the standard 12-lead surface ECG were 23 times more likely to fail. Whilst it is argued that the TWI itself should not result in screening failure, associated changes in the ST segment and T wave morphology, such that the T peak occurs later than with an upright T-wave, are likely to be the culprits. Other less common reasons include very large or very small QRS complexes, in which the QRS complexes exceed the absolute sensing limits of the algorithm or are too small to be detected respectively. It certainly highlights the need for improvements to the sensing algorithm of S-ICDs to make it a more viable option for patients prone to over-sensing. Indeed, Kooiman et al. (18) have shown that inappropriate shocks can be reduced by reprogramming the sensing vector and/or the therapy zones using exercise-derived templates, which is consistent with reports from the START study (8).
Inappropriate shock therapy
Although MADIT II has shown that prophylactic ICD implantation improved outcomes post-myocardial infarction with reduced ejection fraction, inappropriate shocks remain a significant burden. Using fairly stringent criteria, one analysis has shown that one or more inappropriate shocks occurred in 83/719 patients (11.5%) of the MADIT II cohort, which represented 184/590 patients (31.2%) of all shock episodes. Inappropriate therapy in total, that is shock or anti-tachycardia pacing (ATP), occurred in 100/719 patients (13.9%) of whom 17 experienced inappropriate ATP without having at least one inappropriate shock. Atrial fibrillation, SVT and abnormal sensing were the commonest causes for inappropriate therapy (44%, 36% and 20%, respectively). The stability detection algorithm was found to be programmed less frequently in patients receiving inappropriate shocks (17% vs. 36%, P=0.030). Importantly, inappropriate shocks and not inappropriate ATP were associated with a greater likelihood of all-cause mortality (hazard ratio 2.29, P=0.025) (16). Another recent post-hoc analysis of the MADIT-CRT cohort demonstrated 4-year cumulative probability for appropriate and in appropriate shocks at 13% and 6% respectively. Moreover, those patients who received appropriate shocks demonstrated an increased risk of mortality [hazard ratio 2.3 (1.47-3.54), P<0.001]. Even after factoring for echocardiographic remodelling, this risk remained elevated (hazard ratio 2.8, P=0.001), suggesting that structural myocardial disease is in itself a predisposition for shock therapy, albeit in an appropriate context, and that the direct mechanical, arrhythmic, or haemodynamic adverse effect of shock therapy may contribute to further structural damage thereby creating a vicious cycle. Importantly, however, appropriate ATP was not associated with increased mortality and that brief episodes of arrhythmia may have activated ATP, offsetting the need for shock (19).
S-ICD technology, interestingly, has been shown to be advantageous in limiting inappropriate spontaneous shocks. In a cohort of 226 subjects from the IDE trial with dual zone programming and 88 subjects with single zone programming, the 2-year inappropriate shock-free rates were 89.7% vs. 73.6% in the dual and single zone programming subgroups respectively (hazard ratio, 0.38, P=0.001). There was no significant difference between groups regarding the delivery of appropriate shocks (20). This data suggests that the active discrimination algorithm regarding QRS complex and T wave morphology was efficacious, not associated with adverse consequences and supports the use of dual- rather than single-zone programming. Indeed, the rates of inappropriate shocks are comparable between S-ICDs and TV-ICDs. Of note, the S-ICD has shown to have greater specificity for discriminating SVT arrhythmias compared to TV-ICDs (98% S-ICD vs. 76.7% single-chamber T-ICD vs. 68% dual-chamber T-ICD) (8).
The possibility of shock-induced myocardial damage and subsequent adverse outcomes are other matters of longstanding concern (21-24), particularly as the S-ICD utilises a higher (80 J) shock compared to TV-ICDs. However, biomarkers indicative of myocardial damage from animal models suggest that the shocks from S-ICDs cause less insult than do TV-ICDs (25), although it remains to be proven that shocks delivered via TV-ICDs cause myocardial damage sufficient to translate into additional risk. Should evidence for this emerge, S-ICDs could be used in preference to TV-ICDs in patients without indications for pacing. On the other hand, patients who have experienced shocks for VT/VF have shown to have a greater mortality than those who do not (13,26,27). This is contradictory to RCT data that demonstrates reductions in all-cause mortality with ICDs. One explanation for this apparent contradiction is that patients who receive shocks have a greater background risk of potentially fatal arrhythmias in the first instance, and that ICDs implanted in this group at least partly mitigate, rather than confer, this risk. Furthermore, inappropriate shocks are often delivered when arrhythmias occur in circumstances that are otherwise independently associated with adverse outcomes, for example AF and heart failure (28,29). There is data demonstrating ICD shocks delivered for sinus tachycardia, over-sensing, or artefact are not associated with excess mortality, in contrast to ICD shocks for true arrhythmias which were associated with higher mortality (30). This supports the notion that abnormal rhythms & not the ICD shock confer risk to the patient. Furthermore, attempts to reduce shocks such as ICD programming to incorporate ATP have not demonstrated a reduction in mortality either (31,32).
Anti-tachycardia pacing (ATP) and delayed therapy
The lack of ATP function in the S-ICD is a contentious one. ATP was incorporated into ICDs to avoid shocks in the context of monomorphic VT. MADIT II, which has demonstrated sustained long term benefit of ICD therapy (10) has also demonstrated a 40% cumulative probability of appropriate ICD therapy (ATP or shock) for VT/VF in a 4-year period post-ICD implantation. Another study showed that in patients who had received an ICD following a myocardial infarction and had a history of malignant ventricular arrhythmias, 52% had ventricular arrhythmias, the majority of which (670/671 events) were sustained monomorphic VT for which ATP had an effectiveness of 96% (men follow-up approx., 2 years) (33). In ischaemic cardiomyopathy patients with systolic dysfunction (LVEF <35%) secondary to myocardial infarction, the efficacy rate of ATP for VT termination has been shown to be as high as 93%. Of a total of 214 episodes of VT, only 3% were associated with no change in VT, 2% an increase in rate of VT, 2% therapeutic exhaustion and 1% with syncope (34). Although this is also desirable for secondary prevention in patients who have experienced VT in the past, it is not necessarily a mandatory feature of an ICD device, not least in patients at low risk of developing such arrhythmia in the first instance and much less for recurrent VT. The SCD-HeFT study showed that over almost three years of follow-up, only a third of patients with VT had more than one episode, which translated to 1.8% annual risk (21,35). In one cohort of patients it was shown that 55.5% (95% CI, 52.0-59.0%) of single or dual chamber ICDs recipients did not require pacing or receive ATP after 5 years and therefore could have been eligible to receive an S-ICD instead. Patients who had ICDs for secondary prevention, severe heart failure and prolonged QRS duration were more likely to need pacing, and therefore were unlikely candidates for S-ICD (36). Furthermore, ATP is not without risk; it is possible for ATP to accelerate VT to polymorphic VT or VF (37-39) which has been associated with a higher mortality risk (5). A longer time to detect and deliver a shock also affords greater leniency for VT events to self-terminate, which would otherwise be treated with a shock and categorised as “appropriate” therapy. An analysis of rapid-rate NSVT during routine ICD interrogation in patients with heart failure showed that these episodes were polymorphic in 23% of patients (11). The Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (Pain-FREE Rx II) trial (40) showed that patients randomised to receive shock-only, 34% of fast VT episodes terminated spontaneously before therapy, suggesting that a considerable proportion of ATP intervention, which is delivered before a shock, may be unnecessary. As discussed previously, however, ATP has not been associated with increased mortality (16,19).
Other concerns regarding the S-ICDs compared to TV-ICDs relate to the delays in the initiation and delivery of therapy, due to the more prolonged detection algorithm and charge time to deliver 65-80 J in the S-ICD. Data taken from Massachusetts General Hospital demonstrated that time to therapy in three models of TV-ICDs (St Jude Medical, n=117; Boston Scientific, n=61; Medtronic, n=116) was 7.1±1.6 s (mean ± SD), compared to S-ICD time of 14.6±2.9 s. Of interest, the TV-ICD 5-95% range was narrow at 2.25-7.55 s, compared to the positively skewed distribution with S-ICD extending to >24 s time to therapy (41). A total of 88% of tests had times to delivery of less than 18 s and 95% of episodes less than 21 s. Considering the fact that S-ICD discharge time is approximately 7 s, this suggests that arrhythmia detection can take at least 10 s in more than 10% of patients with S-ICD. It is argued that the length of delay in sensing and delivering a therapeutic shock with S-ICD is unacceptably prolonged compared to TV-ICDs. For example, although the RELEVANT study reported a 6 second increase in the detection time compared to controls when programmed at 12 of 16 beats (42), the longer detection times reported in these studies are also not as prolonged as the higher range demonstrated with the S-ICD. Furthermore, MADIT-RIT showed that increasing the standard 1 s delay to 2.5 s in the VF zone would increase the time to therapy by 1.5 s, which is still considerably shorter than the durations observed with S-ICD (43). However, longer times to therapy may reduce the number of unnecessary shocks. For example, the IDE study demonstrated that annual mortality of S-ICDs was 3.7%, which is less than shown in previous clinical studies, suggesting that a longer time to shock may result in fewer adverse consequences (44). Another demonstrated that the delay in therapy delivery is not associated with increased episodes of syncope (40), although this was in the context of fast VT rather than VF and the time to shock was less than the lag seen with S-ICDs. Nevertheless, MADIT-RIT did demonstrate that, over an average follow-up of 1.4 years, high-rate and delayed ICD therapy were associated with significant reductions in first occurrence of inappropriate therapy (hazard ratio high-rate vs. conventional therapy 0.21; 95% CI, 0.13-0.34; P<0.001; hazard ratio delayed vs. conventional therapy, 0.24; 95% CI, 0.15-0.40; P<0.001). Moreover, high-rate and delayed therapy was also associated with a reduction in all-cause mortality (hazard ratio high-rate vs. conventional therapy, 0.45; 95% CI, 0.24-0.85; P=0.01; hazard ratio delayed vs. conventional therapy, 0.56; 95% CI, 0.30-1.02; P=0.06) (43). It supports the notion that simpler programmes are preferable to complicated algorithms. The S-ICD mimics this programming system by providing high-rate zones of therapy and prolongation of detection-to-shock time to reduce (inappropriate) shock therapy. The IDE trial (2) also showed that time to therapy for appropriate shocks fell within the range of the prolonged detection time shown to be beneficial in MADIT-RIT (43). Of course, although it is possible that the benefits of delayed therapy are due to the benign and/or self-terminating nature of the arrhythmias rather than the lack of therapy, it is accepted that malignant arrhythmias such as VF necessitate prompt intervention. Indeed, delays in shock therapy delivery in these instances can be life-threatening; one report suggested that two out of three inappropriate shocks for VF had delays equivalent to 24 and 27 s. Unsurprisingly, both were associated with syncope (9). In order to mitigate these delays in therapy a new VF detection algorithm employing more rapid slopes in auto-gain thresholding profile to detect fine VF have been added in order to avoid under-detecting a transition from SVT/VT to fine VF.
Minimising under-sensing of VT/VF
Another as yet unanswered question, relates to the characteristics that might predict which patients are at risk for under-sensing VF with consequent delays in therapy, feasibility of developing bespoke sensing programmes for individual patients, and the threshold at which delays influence successful defibrillation. Indeed, 2% of subjects in the IDE trial had unsuccessful S-ICD implants as a result of incomplete or unsuccessful VF conversion testing, 10 were unable to complete the VF conversion testing protocol, and a total of 11 received more than one failed shock (2). Another recent study reports a 10% defibrillation test failure rate for S-ICDs, which although comparable to the control group (3), was nevertheless greater than previously shown for TV-ICDs (45). It is also unclear if the factors that influence DFT (46) are also applicable to S-ICDs, and as such the evidence-base for S-ICDs, in comparison to TV-ICDs, is currently lacking.
Complications
The S-ICD is not immune from the complications that plague the conventional ICD; infection and suboptimal lead position. Nevertheless, the 180-day complication-free rate relating to the device, labelling and the insertion procedure in the IDE study was 92.1% with a lower confidence limit of 88.9% that was above the pre-specified performance goal. EFFORTLESS reported 15 system related complications in 14 patients (3%) occurred in the first 30 days post implant, equating to a peri-operative complication-free rate of 97%. At 360 days post implantation, the documented system or implantation-related complication-free rate was 94%. Infection rates were 5.7% and 4% in the IDE and EFFORTLESS studies respectively, and 1.3% of cases required exploration in IDE and 2.2% in EFFORTLESS. Nevertheless, complication rates have been shown to improve with time and experience, and by optimising screening for T wave over-sensing on exercise, using a suture sleeve to prevent lead migration and reductions in implant time (47).
Eligibility for S-ICD
The indication for an ICD is not uncommonly associated with other significant cardiac morbidity, such as heart failure, low ejection fraction, or bundle branch block that may necessitate cardiac resynchronisation therapy or pacing. With studies demonstrating ICD upgrades to CRT-D ranging between 5% and 42% (48-50), the use of S-ICD will be naturally restricted to patients who do not have or are unlikely to develop a pacing indication. Furthermore, the lack of ATP with S-ICD necessitates careful consideration of its use in patients with a history of monomorphic VT. The subjects in whom S-ICD represents a simple alternative to TV-ICD are those that have difficult venous access or are otherwise immunocompromised, to minimise infection risk (51). Young patients who are at increased risk of long-term complications associated with repeated venous access and those at risk of sudden cardiac death, for example, patients with channelopathies such as Brugada and long QT syndrome, may also benefit from S-ICDs. Despite the fact that the characteristic QRS- and T-wave morphology of Brugada syndrome confers an additional risk of double-counting and inappropriate therapy, case reports have demonstrated successful implantation and clinical efficacy of S-ICD to terminate VF without adverse sequelae in this population, provided that appropriate pre-implant screening is conducted with due diligence beforehand (52). On the other hand, inappropriate shock may be more common due to supraventricular tachyarrhythmias in Brugada syndrome, particularly AF, but this will become clearer with more prolonged follow-up (53). Of course, in light of the above, the question remains whether the S-ICDs is as efficacious as the TV-ICD especially when considering its longer duration to therapy and tendencies for over-sensing T waves. Nevertheless, there is evidence to suggest the increasing uptake of S-ICD primary prevention in patients with cardiomyopathy and secondary prevention in those with history of VT/VF (47). It is therefore argued that the S-ICD is a reasonable alternative to conventional ICD for primary prevention in relatively well patients as it is sufficient to rescue a patient in a time of need without the disadvantages associated with intravenous leads.
Future directions
Whilst the S-ICD represents an improvement on the degree of invasiveness associated with inserting TV-ICDs, there is much potential for further progress. This is best exemplified by the development of Nanostim™ (St. Jude Medical), a small cylindrical device smaller than an AAA battery, which is the first self-contained leadless pacemaker (LCP) which can be implanted percutaneously (54,55). This device may ultimately obviate conventional pacing technologies and enhance arrhythmia sensing by the S-ICD which will be able to incorporate intracardiac electrogram data to confirm VT as opposed to SVT as in conventional dual chamber ICDs. With advances in battery technology and redesign of the current S-ICD, the volume of the generator is being reduced which will increase comfort and suitability for paediatric patients. The algorithms being utilised by the system are constantly being reviewed and revised with analysis of events from the IDE & EFFORTLESS cohorts. A significant step will be to incorporate data from all three lead vectors to automatically minimise T wave oversensing, for example when required on exercise to avoid inappropriate shocks. It is conceivable that remote monitoring will also enable optimisation of follow-up and detection of transient oversensing enabling prospective reprogramming to prevent inappropriate shocks and manage subclinical arrhythmias such as AF and NSVT accordingly.
Conclusions
The S-ICD has demonstrated efficacy and promising clinical value as an alternative to conventional ICDs, particularly in the younger patients by obviating the long term risks associated with transvenous leads. Initial trials have shown that it has comparable defibrillation success rates and inappropriate shock delivery to conventional models, with improvements in technology already being implemented, especially with the use of dual zone and exercise-based programming. Nevertheless, results from the (prospective, randomised comparison of subcutaneous and transvenous implantable cardioverter-defibrillator therapy-PRAETORIAN) trial (NCT01296022) are keenly awaited to robustly evaluate the S-ICD with respect to inappropriate shocks and complication rates (56). The long term role of S-ICDs in managing inherited cardiac arrhythmias is as yet unclear although initial studies have also been promising. As with the conventional ICD, it is envisaged that subsequent generations of the S-ICD models will be less cumbersome and a wireless model will enable remote follow-up. Of course, although therapy should be tailored to an individual’s risk and it is not envisaged that the S-ICD will completely replace TV-ICDs in its current form, the S-ICD is establishing its role as a viable alternative particularly as primary prevention in non-pacing dependent younger patients, and should be embraced as a valuable addition in our ability to reduce sudden cardiac death.
Acknowledgements
Disclosure: Dr. Lambiase receives educational grants and speaker fees from Boston Scientific and educational grants from Medtronic. He is supported by UCLH Biomedicine NIHR.
References
- Lambiase PD, Barr C, Theuns DA, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J 2014;35:1657-65. [PubMed]
- Weiss R, Knight BP, Gold MR, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation 2013;128:944-53. [PubMed]
- Köbe J, Reinke F, Meyer C, et al. Implantation and follow-up of totally subcutaneous versus conventional implantable cardioverter-defibrillators: a multicenter case-control study. Heart Rhythm 2013;10:29-36. [PubMed]
- Knops RE, Olde Nordkamp LR, de Groot JR, et al. Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2013;10:1240-3. [PubMed]
- Saxon LA. The subcutaneous implantable defibrillator: a new technology that raises an existential question for the implantable cardioverter-defibrillator. Circulation 2013;128:938-40. [PubMed]
- Lambiase PD, Srinivasan NT. Early experience with the subcutaneous ICD. Curr Cardiol Rep 2014;16:516. [PubMed]
- Olde Nordkamp LR, Warnaars JL, Kooiman KM, et al. Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRS-T-wave morphology screening. J Cardiovasc Electrophysiol 2014;25:494-9. [PubMed]
- Gold MR, Theuns DA, Knight BP, et al. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: the START study. J Cardiovasc Electrophysiol 2012;23:359-66. [PubMed]
- Jarman JW, Todd DM. United Kingdom national experience of entirely subcutaneous implantable cardioverter-defibrillator technology: important lessons to learn. Europace 2013;15:1158-65. [PubMed]
- Goldenberg I, Gillespie J, Moss AJ, et al. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation 2010;122:1265-71. [PubMed]
- Chen J, Johnson G, Hellkamp AS, et al. Rapid-rate nonsustained ventricular tachycardia found on implantable cardioverter-defibrillator interrogation: relationship to outcomes in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial). J Am Coll Cardiol 2013;61:2161-8. [PubMed]
- Schaechter A, Kadish AH; DEFibrillators In Non-Ischemic Cardiomyopathy Treatment Evaluation. DEFibrillators In Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE). Card Electrophysiol Rev 2003;7:457-62. [PubMed]
- Saxon LA, Hayes DL, Gilliam FR, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359-67. [PubMed]
- Poole JE, Gold MR. Who should receive the subcutaneous implanted defibrillator?: The subcutaneous implantable cardioverter defibrillator (ICD) should be considered in all ICD patients who do not require pacing. Circ Arrhythm Electrophysiol 2013;6:1236-44; discussion 1244-5. [PubMed]
- Piccini JP, Al-Khatib SM, Hellkamp AS, et al. Mortality benefits from implantable cardioverter-defibrillator therapy are not restricted to patients with remote myocardial infarction: an analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Heart Rhythm 2011;8:393-400. [PubMed]
- Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol 2008;51:1357-65. [PubMed]
- Groh CA, Sharma S, Pelchovitz DJ, et al. Use of an electrocardiographic screening tool to determine candidacy for a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2014;11:1361-6. [PubMed]
- Kooiman KM, Knops RE, Olde Nordkamp L, et al. Inappropriate subcutaneous implantable cardioverter-defibrillator shocks due to T-wave oversensing can be prevented: implications for management. Heart Rhythm 2014;11:426-34. [PubMed]
- Sood N, Ruwald AC, Solomon S, et al. Association between myocardial substrate, implantable cardioverter defibrillator shocks and mortality in MADIT-CRT. Eur Heart J 2014;35:106-15. [PubMed]
- Gold MR, Weiss R, Theuns DA, et al. Use of a discrimination algorithm to reduce inappropriate shocks with a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2014;11:1352-8. [PubMed]
- Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009-17. [PubMed]
- Larsen GK, Evans J, Lambert WE, et al. Shocks burden and increased mortality in implantable cardioverter-defibrillator patients. Heart Rhythm 2011;8:1881-6. [PubMed]
- Deyell MW, Qi A, Chakrabarti S, Yeung-Lai-Wah JA, et al. Prognostic impact of inappropriate defibrillator shocks in a population cohort. Heart 2013;99:1250-5. [PubMed]
- Raitt MH. Reducing shocks and improving outcomes with implantable defibrillators. JAMA 2013;309:1937-8. [PubMed]
- Killingsworth CR, Melnick SB, Litovsky SH, et al. Evaluation of acute cardiac and chest wall damage after shocks with a subcutaneous implantable cardioverter defibrillator in Swine. Pacing Clin Electrophysiol 2013;36:1265-72. [PubMed]
- Moss AJ, Greenberg H, Case RB, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004;110:3760-5. [PubMed]
- Saxon LA, Bristow MR, Boehmer J, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation 2006;114:2766-72. [PubMed]
- Swedberg K, Olsson LG, Charlesworth A, et al. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur Heart J 2005;26:1303-8. [PubMed]
- Olsson LG, Swedberg K, Ducharme A, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol 2006;47:1997-2004. [PubMed]
- Powell BD, Saxon LA, Boehmer JP, et al. Survival after shock therapy in implantable cardioverter-defibrillator and cardiac resynchronization therapy-defibrillator recipients according to rhythm shocked. The ALTITUDE survival by rhythm study. J Am Coll Cardiol 2013;62:1674-9. [PubMed]
- Saxon LA, Varma NJ, Lindenfeld J, et al. ICD programming trends and relationship to survival: the Altitude Study Group. Heart Rhythm 2013;10:1420-1.
- Ha AH, Ham I, Nair GM, et al. Implantable cardioverter-defibrillator shock prevention does not reduce mortality: a systemic review. Heart Rhythm 2012;9:2068-74. [PubMed]
- Mont L, Valentino M, Sambola A, et al. Arrhythmia recurrence in patients with a healed myocardial infarction who received an implantable defibrillator: analysis according to the clinical presentation. J Am Coll Cardiol 1999;34:351-7. [PubMed]
- Oliveira M, Antunes E, da Silva N, et al. Antitachycardia pacing in patients with left ventricular dysfunction and hemodynamically unstable arrhythmias. Rev Port Cardiol 1999;18:161-6. [PubMed]
- Hanna R, Hellkamp A, Mark D, et al. Predictors of ventricular tachyarrhythmias treated with the ICD in the sudden cardiac death in heart failure trial. Poster, ESC Congress 2012. Munich, Germany, August 26, 2012.
- de Bie MK, Thijssen J, van Rees JB, et al. Suitability for subcutaneous defibrillator implantation: results based on data from routine clinical practice. Heart 2013;99:1018-23. [PubMed]
- Sweeney MO, Wathen MS, Volosin K, et al. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: results from the Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial. Circulation 2005;111:2898-905. [PubMed]
- Wathen MS, Sweeney MO, DeGroot PJ, et al. Shock reduction using antitachycardia pacing for spontaneous rapid ventricular tachycardia in patients with coronary artery disease. Circulation 2001;104:796-801. [PubMed]
- Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003;289:2685-94. [PubMed]
- Wathen MS, DeGroot PJ, Sweeney MO, et al. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx II) trial results. Circulation 2004;110:2591-6. [PubMed]
- Rav Acha M, Milan D. Who should receive the subcutaneous implanted defibrillator?: Timing is not right to replace the transvenous implantable cardioverter defibrillator. Circ Arrhythm Electrophysiol 2013;6:1246-51; discussion 1251. [PubMed]
- Gasparini M, Menozzi C, Proclemer A, et al. A simplified biventricular defibrillator with fixed long detection intervals reduces implantable cardioverter defibrillator (ICD) interventions and heart failure hospitalizations in patients with non-ischaemic cardiomyopathy implanted for primary prevention: the RELEVANT [Role of long dEtection window programming in patients with LEft VentriculAr dysfunction, Non-ischemic eTiology in primary prevention treated with a biventricular ICD] study. Eur Heart J 2009;30:2758-67. [PubMed]
- Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275-83. [PubMed]
- Gasparini M, Proclemer A, Klersy C, et al. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA 2013;309:1903-11. [PubMed]
- Russo AM, Sauer W, Gerstenfeld EP, et al. Defibrillation threshold testing: is it really necessary at the time of implantable cardioverter-defibrillator insertion? Heart Rhythm 2005;2:456-61. [PubMed]
- Swerdlow CD, Russo AM, Degroot PJ. The dilemma of ICD implant testing. Pacing Clin Electrophysiol 2007;30:675-700. [PubMed]
- Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, et al. The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol 2012;60:1933-9. [PubMed]
- Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539-49. [PubMed]
- Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329-38. [PubMed]
- Scott PA, Whittaker A, Zeb M, et al. Rates of upgrade of ICD recipients to CRT in clinical practice and the potential impact of the more liberal use of CRT at initial implant. Pacing Clin Electrophysiol 2012;35:73-80. [PubMed]
- Tompkins C, McLean R, Cheng A, et al. End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol 2011;22:1099-104. [PubMed]
- De Maria E, Cappelli S, Cappato R. Shock efficacy of the entirely subcutaneous defibrillator for termination of spontaneous ventricular fibrillation in Brugada syndrome. Heart Rhythm 2013;10:1807-9. [PubMed]
- Rosso R, Glick A, Glikson M, et al. Outcome after implantation of cardioverter defibrillator [corrected] in patients with Brugada syndrome: a multicenter Israeli study (ISRABRU). Isr Med Assoc J 2008;10:435-9. [PubMed]
- Koruth JS. Feasibility and Efficacy of Percutaneously Delivered Leadless Cardiac Pacing in an In Vivo Ovine Model. Presented at Heart Rhythm 34th Annual Scientific Sessions. Denver, Colorado, USA. 2013. Available online: http://ondemand.hrsonline.org/Common/presentation-detail.aspx/8/23/1072/7301
- Reddy VY, Knops RE, Sperzel J, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation 2014;129:1466-71. [PubMed]
- Olde Nordkamp LR, Knops RE, Bardy GH, et al. Rationale and design of the PRAETORIAN trial: a Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am Heart J 2012;163:753-760.e2.


