Shone’s anomaly: a report of one case in sub-Saharan Africa
Introduction
Shone’s anomaly, is a rare “congenital” cardiac malformation, of the left heart and aortic arch including in its complete form a supravalvular mitral ring, parachute mitral valve, subaortic stenosis, and aortic coarctation (1,2). There have been few reports of this anomaly with most originating from high income countries. This anomaly is traditionally reported in infants and children and very rarely in young adults where the clinical presentation and post-surgical prognosis may have distinctive features (3,4). Africa has a high burden of congenital heart disease. The huge progress made in the developed regions of the world in diagnostic options and surgical and interventional management of complex congenital heart diseases have not been reproduced in Africa (5,6). This case illustrates the challenges of diagnosis and management of Shone anomaly in areas with limited access to cardiac echocardiography
Case report
History
A 17-year-old male Cameroonian was referred in November 2007 to the cardiology unit of the Yaoundé Teaching Hospital for the investigation of a cardiac murmur. A year prior to presentation, he started to complain of chest discomfort. There was no palpitation, legs swelling, paroxysmal nocturnal dyspnea or dizziness. Two weeks before presentation, he started to complain of a class II dyspnea of the New York Heart Association (NYHA) functional classification, heralded by upper respiratory tract infection symptoms.
Physical examination
His general appearance was unremarkable with no particular dysmorphic features. His pulse was regular, and slightly stronger on the left radial and humeral arteries and generally on upper limbs. Blood pressure level was 120/80, 130/90 and 110/70 mmHg on the left arm, right arm and lower limbs respectively. The 1st heart sound was muffled and the second was not audible. There was a harsh 4/6 ejection systolic murmur, best heard at the aortic area, radiating to the neck. Jugular venous pressure was normal. He had no peripheral oedema and no ascites. The remaining of physical examination was unremarkable.
Cardiac echography
Echocardiographic studies showed a sub-valvular aortic stenosis with estimated area at 1.9 cm2 (Figure 1). There was also an aortic coarctation with a peak systolic velocity and pressure of 4.61 m/s and 85.01 mmHg respectively (Figure 2). There was a parachute deformity of the mitral valve (Figure 3), producing a reduction in its opening and mitral stenosis (mean transmitral gradient of 6.5 mmHg, mitral valve surface area by Hattle formula of 2.6 and 2.8 mm2 by planimetry). There was no thickening of the mitral annulus. The left ventricle was hypertrophied. The left atrium was dilated (antero-posterior diameter of 67 mm) (Figure 1). There was no atrial or ventricular septal defect. The diagnosis of left heart and aortic arch obstruction was made.
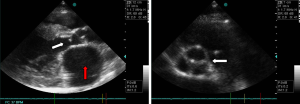
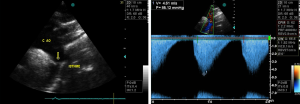
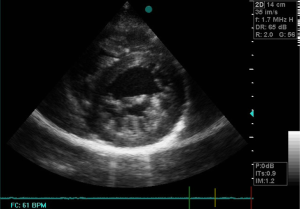
Other tests
The chest X-ray was normal. The electrocardiogram (ECG) showed a sinus rhythm, left ventricular hypertrophy by Sokolow-Lyon criterion and non-specific repolarisation abnormalities. Full blood count, erythrocyte sedimentation rate and C-reactive protein were normal.
Brief surgical management and outcome
Patient uneventfully underwent a percutaneous angioplasty of the aortic coarctation and a sternotomy with a resection of the subaortic membrane. The mitral valve abnormality was not corrected. On post-operative evaluation, he had NYHA class I dyspnoea. He developed a new atrial fibrillation. Blood pressure was 120/70 mmHg on both upper and lower limbs. There was a residual aortic systolic murmur graded 2/6. Echocardiographic evaluation showed a normal mean transvalvular aortic gradient of 3.4 mmHg and a complete absence of the sub-aortic stenosis. The aortic stent was functional with mean gradient of 18.4 mmHg.
Discussion
Shone’s anomaly is a very rare entity which is diagnosed most frequently in its incomplete form. Our patient had sub-aortic stenosis, aortic coarctation and a parachute mitral valve. There was no other associated congenital anomaly. To the best of our knowledge, this is the second report of this rare congenital abnormality from sub-Saharan Africa (SSA). Although very rare, the apparent lower reported cases of Shone anomaly in SSA may reflect the limited access to medical care as well as the paucity of human and logistic resources. Takawira et al. reported an adult with Shone’s anomaly, bicuspid aortic valve, patent ductus arteriosus and pulmonary hypertension (7). The prognosis of this anomaly depends of the complexity and the severity of the different obstructive lesions (1,3,8). Shone’s anomaly in most cases is diagnosed during the neonatal period or before adolescence (8,9), probably reflecting a higher level of awareness, access to medical care, and availability of human resources and echocardiographies in high income countries. The disease was diagnosed in our patient at the age of 17, a distinctive feature reported in some cases (4,10,11). While the condition may be fatal before adult age, we hypothesized that our patient had survived into adulthood probably because of the mild degree of mitral stenosis. It should be noted that our patient was only diagnosed when he became overtly symptomatic (as a result of a probable increased cardiac demand after a flu like episode) as in most cases diagnosed late. For instance, Popescu et al. (12) described a case of Shone’s anomaly in a 19-year-old girl who also had bi-atrial enlargement, bicuspid aortic valves and dysplastic mitral valves causing mitral stenosis and severe pulmonary hypertension. In line with our observation and that of Popescu, Prunier et al. reported on a 33-year-old man who presented with atrial fibrillation, with an enlarged atrium leading to echocardiographic diagnosis of Shone’s anomaly (13).
With regards to the treatment and outcome, our patient underwent a sequential repair of the aortic stenosis percutaneously, followed by a resection of the subaortic membrane but no intervention was performed on the mitral abnormality. Mitral valve obstruction may be the most critical abnormality determining the overall prognosis, with the severity of the obstruction determining the long-term outcome (3). The mitral valve was not repaired in our patient probably because the lesion was not severe. The patient had no evidence of recurrence on his most recent echo performed 2 years after his initial surgery. In line with this approach, a large surgical series including 28 children with Shone’s anomaly, St Louis et al. reported that deferring mitral valve repair does not worsen the prognosis of the patient (14). Similarly Ikemba et al. (9) indicated that in the management of Shone’s anomaly, mitral valve repair in patients with parachute mitral valve and subaortic stenosis is increasingly required in the absence of aortic coarctation. All together these reports and our case suggest that surgical management of Shone anomaly in young adults may not be different from that in paediatric age patients.
Limitations
We are aware of the major limitation of this report pertaining to its retrospective nature which did not allow us to obtain detailed information on the echocardiogram such as the dimensions of the left ventricular outflow tract, the supravavular area, the precise location of the coarctation, the two dimensional measurements of the aortic arch, isthmus, coarcted segment and the aorta below the coarctation as well as spectral and color Doppler characteristics of the coarctation. Our report also has major strength including the fact that it confirms that the anomaly occurs in a wide variety of geographical settings. In addition, the current case support the hypothesis that prolonged symptoms-free survival correlates with severity of mitral stenosis. This report complements previous reports from high income countries regarding the general agreement that management of mitral valve abnormalities can be delayed and may not be necessary in the presence of aortic coarctation.
Conclusions
This case report raises the awareness about this rare syndrome and highlights the importance of cardiac ultrasonography in resource limited settings. Widespread use of ultrasonography might have led to an early diagnosis of this condition in this patient.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Shone JD, Sellers RD, Anderson RC, et al. The developmental complex of “parachute mitral valve,” supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol 1963;11:714-25. [PubMed]
- Bolling SF, Iannettoni MD, Dick M 2nd, et al. Shone’s anomaly: operative results and late outcome. Ann Thorac Surg 1990;49:887-93. [PubMed]
- Delmo Walter EM, Komoda T, Siniawski H, et al. Long-term surgical outcome of mitral valve repair in infants and children with Shone’s anomaly. Eur J Cardiothorac Surg 2013;43:473-81; discussion 481-2. [PubMed]
- Grimaldi A, Vermi AC, Maisano F, et al. Echocardiographic patterns of incomplete Shone’s syndrome in adults. J Heart Valve Dis 2011;20:552-6. [PubMed]
- Mocumbi AO, Lameira E, Yaksh A, et al. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol 2011;148:285-8. [PubMed]
- Mocumbi AO. The challenges of cardiac surgery for African children. Cardiovasc J Afr 2012;23:165-7. [PubMed]
- Takawira FF, Sinyangwe G, Mwangi MN, et al. Shone’s complex variant associated with a patent ductus arteriosus: Simultaneous treatment of coarctation and patent ductus arteriosus using a covered stent. SA Heart 2010;17:282-6.
- Brown JW, Ruzmetov M, Vijay P, et al. Operative results and outcomes in children with Shone’s anomaly. Ann Thorac Surg 2005;79:1358-65. [PubMed]
- Ikemba CM, Eidem BW, Fraley JK, et al. Mitral valve morphology and morbidity/mortality in Shone’s complex. Am J Cardiol 2005;95:541-3. [PubMed]
- Moustafa SE, Lesperance J, Rouleau JL, et al. A forme fruste of Shone’s anomaly in a 65 year-old patient. Mcgill J Med 2008;11:19-21. [PubMed]
- Koelble N, Weiss BM, Wisser J, et al. Shone’s anomaly complicated by ascending aortic aneurysm in a pregnant woman. J Cardiothorac Vasc Anesth 2001;15:84-7. [PubMed]
- Popescu BA, Jurcut R, Serban M, et al. Shone’s syndrome diagnosed with echocardiography and confirmed at pathology. Eur J Echocardiogr 2008;9:865-7. [PubMed]
- Prunier F, Furber AP, Laporte J, et al. Discovery of a parachute mitral valve complex (Shone’s anomaly) in an adult. Echocardiography 2001;18:179-82. [PubMed]
- St Louis JD, Bannan MM, Lutin WA, et al. Surgical strategies and outcomes in patients with Shone complex: a retrospective review. Ann Thorac Surg 2007;84:1357-62; discussion 1362-3. [PubMed]


