Ischemic conditioning: the challenge of protecting the diabetic heart
Cardiovascular disease (CVD) remains the leading cause of death in the United States and globally, with acute myocardial infarction (AMI) accounting for a substantial portion of CVD-associated mortality (1). Timely reperfusion is the standard clinical therapy for AMI and, although necessary to rescue ischemic tissue, restoration of blood flow can paradoxically exacerbate cell death (rather than initiate salvage) in populations of ischemic myocytes, a phenomenon termed lethal ischemia-reperfusion (I/R) injury (2-6). The volume of myocardium rendered necrotic following I/R (i.e., myocardial infarct size) is a primary determinant of mortality and morbidity associated with AMI (6-8). Indeed, decades of preclinical and clinical investigation have been devoted to: (I) elucidating the mechanisms of I/R injury, and (II) developing mechanisms-based therapies to augment the benefits of early reperfusion and reduce myocardial infarct size. Despite this substantial investment of time and resources, no advances have, to date, been successfully translated into clinical practice (8-10).
In 1986, Murry et al. made the landmark observation that the heart could be ‘preconditioned’ or rendered resistant to lethal I/R injury, by exposure to a brief and non-lethal, antecedent ischemic insult (11). Subsequent studies expanded the paradigm of myocardial ‘conditioning’ beyond the phenomenon of ischemic preconditioning to encompass postconditioning and remote conditioning (5,12,13). Overwhelming experimental evidence, obtained in multiple models and species, has demonstrated that all three forms of myocardial conditioning induce potent cardioprotection (5,6,9). However, the vast majority (>90%) of these studies have been conducted using healthy, juvenile or adult populations that do not manifest the risk factors and comorbid conditions typically seen in patients with CVD and suffering AMI (14). A major, well-established independent risk factor for CVD and AMI, the prevalence of which has doubled over the past 20 years, is type-2 diabetes (1,15,16), and there is emerging concern that the efficacy of conditioning-induced cardioprotection may be compromised in the diabetic heart (4,14,17-20). Our aim in the current review is to focus on this important issue: we provide a synopsis of our present understanding of the effect of type-2 diabetes on the infarct-sparing effect of ischemic conditioning, and the challenges of limiting I/R injury in the diabetic heart.
What is ischemic conditioning?
Definitions and basic concepts
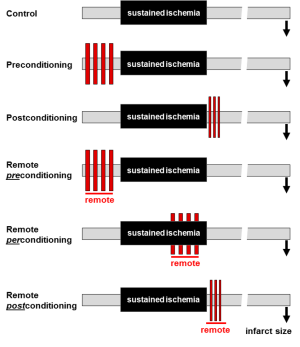
Ischemic conditioning renders the myocardium resistant to I/R injury via the up-regulation of as-yet incompletely understood, endogenous cardioprotective signaling cascades (5,6). There are three permutations of conditioning that differ in terms of the site and timing of the brief ischemic stimulus (Figure 1) (5). Classic ischemic preconditioning, as first described by Murry and colleagues (11), is initiated by subjecting the heart to 2-4 repeated episodes of brief (2-5 minutes) ischemia, interrupted by intervening 5-minute periods of reperfusion, before the onset of a sustained ischemic insult. By contrast, postconditioning is not a pre-ischemic intervention but, rather, as described in the seminal publication by Zhao et al. (12), is a form of modified reperfusion, i.e., blood flow to the ischemic myocardium is restored in a stuttered or staccato manner, with a typical algorithm of 3-6 cycles of (10-30 seconds of reperfusion interspersed with 10-30 seconds periods of re-occlusion), before establishing full and complete reperfusion. For both preconditioning and postconditioning, the protective stimulus (brief antecedent ischemia or stuttered reflow) is applied in heart, at the same site and in the same vascular territory as the sustained period of ischemia. Remote conditioning differs importantly from both of the aforementioned strategies in that cardioprotection is evoked by brief ischemia applied at a distant site, such as a different and distinct myocardial vascular bed [as first reported by Przyklenk et al. (13)] or a remote and less vulnerable tissue or organ such as skeletal muscle (21). With regard to timing, protection with remote conditioning can be achieved when the stimulus is applied before sustained coronary occlusion, during the sustained ischemic insult, or at the time of reperfusion (remote pre-, per- or post-conditioning, respectively; Figure 1) (5).
Reduction of infarct size: the hallmark of ischemic conditioning
Despite these temporal and spatial differences, all three facets of ischemic conditioning share a common and overarching theme: all are profoundly effective in reducing myocardial infarct size beyond that achieved by reperfusion alone (Figure 2) (5,6). Conditioning-induced cardioprotection has, with rare exceptions, been documented and confirmed in countless laboratories and in all models and species that have been tested. There are reports that the benefits of ischemic conditioning (in particular, preconditioning and postconditioning) may extend to other pathophysiologic aspects of I/R injury, including postischemic myocardial ‘stunning’, arrhythmias, microvascular damage and endothelial dysfunction (18,23,24). However, there is no question that the most robust endpoint for the assessment of cardioprotection is infarct size, and the established hallmark of ischemic conditioning is its infarct-sparing effect (5,6).
Cellular mechanisms: the major players
In addition to sharing a common primary endpoint—reduction of infarct size—the three facets of ischemic conditioning also appear to share common elements in terms of cellular mechanisms. Not surprisingly, the greatest insight has been gained into the mechanisms of ischemic preconditioning [comprehensive reviews provided in (4,5,24-27)]. There is a consensus that preconditioning is initiated through ligand-receptor interactions including, most notably, stimulation of Gi-protein coupled receptors. The archetypal trigger for preconditioning, first described in 1991, is release of adenosine from myocardium rendered ischemic during the brief antecedent preconditioning stimulus and binding to adenosine A1 or A3 receptors on the cardiomyocyte membranes (28,29). In the ensuing years, redundancies in the ligands capable of triggering preconditioning via binding to their respective receptors were identified, including (but not limited to) bradykinin, opioids, acetylcholine and TNF-α (30-33). Ligand-receptor binding subsequently activates multiple signaling cascades in a complex, biphasic and possibly redundant manner, following the general paradigm of: (I) initial up-regulation of phosphatidylinositol 3-kinase (PI3 kinase)/Akt, nitric oxide-mediated activation of protein kinase G (PKG) and subsequent activation of the ε isoform of protein kinase C (PKC) during the early minutes of sustained ischemia; and (II) receptor re-population and up-regulation of the so-called reperfusion injury salvage kinase (RISK) and/or survival activating factor enhancement (SAFE) pathways during the early seconds-minutes following restoration of blood flow (27,34-36). There appears to be minimal overlap or intersection between these latter two reperfusion-associated signaling cascades: key components of the RISK pathway include PI3 kinase/Akt, extracellular signal regulated kinase (ERK), p70S6 kinase and glycogen synthase kinase 3β (GSK-3β), while the pivotal constituents of the SAFE pathway are janus activated kinase (JAK) and signal transducer and activator of transcription (STAT) (4,5,24-27,35,36). Nonetheless, both pathways converge on the mitochondria, the proposed end-effector of ischemic preconditioning, with specific molecular targets including the mitochondrial adenosine triphosphate-sensitive potassium (KATP) channel, mitochondrial connexin 43, and, most notably, the mitochondrial permeability transition pore (mPTP) (25,37-40). Cardioprotection is purportedly conferred by a resultant stabilization of mitochondrial membranes (including suppression of mPTP opening) and better maintenance of mitochondrial integrity (4,5,24-27).
The discovery of infarct size reduction with post-conditioning, and observations of a comparable magnitude of cardioprotection with both preconditioning and post-conditioning, provided compelling and provocative evidence that pretreatment—and up-regulation of kinase signaling at the onset of ischemia—is not required to render the heart resistant to I/R injury (12). Moreover, the lack of an additive effect of combined administration of preconditioning + postconditioning suggests that common (or redundant) reperfusion-associated mechanisms may underlie the infarct-sparing effect of the two interventions (12). Indeed, receptor-mediated up-regulation of the RISK and/or SAFE pathways, culminating in stabilization of mitochondria (with an emphasis on inhibition of mPTP opening) are hypothesized to play critical roles in the reduction of infarct size achieved with postconditioning (4,5,18,25,27,41-43).
Perhaps not surprisingly, the three common themes of G-protein coupled receptor stimulation on myocyte membranes, activation of multiple kinases including members of the RISK and/or SAFE pathways, and mitochondria as end-effectors have also been implicated as key mechanistic components of remote conditioning [reviewed in (5,44-47)]. There is, however, an inherently unique aspect of remote conditioning not shared by pre- and postconditioning: the cardioprotective signaling cascades are initiated by communication or transfer of a protective signal from the site of the conditioning stimulus to the heart. Details concerning the identity of the signal(s) and mode of communication remain elusive, but two leading theories are under investigation: remote conditioning may be triggered by blood- or perfusate-borne transport of one or more unknown humoral factors (possibly including a small, <15 kDa hydrophobic molecule) (48-50), and communication via neuronal stimulation (5,44-52). Of note, these two hypotheses are not mutually exclusive, are in all likelihood model-dependent, and, in at least some models, both humoral and neuronal communication may be involved (5,44,45,53,54).
Ancillary and alternative mediators of conditioning-induced cardioprotection
As summarized in the preceding paragraphs, intensive interest and attention has focused on the involvement of RISK and SAFE signaling in the infarct-sparing effect of ischemic pre-, post- and remote conditioning. However, additional and less well-characterized mediators have also been postulated to contribute to conditioning-induced cardioprotection, either in concert with or as possible alternatives to the RISK and SAFE cascades. For example, isoforms of PKC (in particular, PKCε) may play a broader role in conditioning-induced cardioprotection, beyond the well-described early activation following brief preconditioning ischemia: PKC has been implicated as a component of kinase signaling initiated in response to both postconditioning and remote preconditioning (26,55-59). Generation of low, sub-lethal levels of reactive oxygen species (ROS) and signaling via nitric oxide have, similarly, been proposed to integrate with both PKC and the RISK and STAT pathways as mediators of pre- and post-conditioning (27,56,57,59-61).
A long-standing concept that may be relevant to the issue of cardioprotection in diabetic cohorts, particularly for ischemic preconditioning, is that alterations in myocardial metabolism play a causal role. Metabolic hallmarks of cardiac ischemia include the rapid (within seconds-minutes) shift from aerobic to anaerobic metabolism and the resultant, progressive temporal decline in myocardial ATP concentration as ATP synthesis via anaerobic glycolysis is insufficient to meet the diminished, residual energy consumption of the ischemic tissue (62). The first report of infarct size reduction with preconditioning was accompanied by evidence of an increase in metabolic efficiency: i.e., preconditioning slowed myocardial energy demand during the subsequent period of sustained ischemia, thereby attenuating the rate of ATP utilization and reducing the rate of anaerobic glycolysis (11,63,64). Ensuing studies argued against the concept of a cause-and-effect relationship between reduced energy demand during prolonged ischemia and infarct size reduction with preconditioning (65). Nonetheless, there is evidence for a mechanistic link between metabolism and preconditioning-induced cardioprotection. Ischemic preconditioning is associated with an increase in glucose uptake during sustained ischemia, an effect that has been attributed to: (I) co-activation of Akt and AMP-activated protein kinase (AMPK: the ‘metabolic master switch’ that responds to ATP depletion and an increase in the ratio of AMP/ATP); (II) translocation of the glucose transporter protein GLUT4 to the cardiomyocyte surface; and (III) subcellular redistribution of hexokinase to mitochondria, where it phosphorylates and facilitates sequestration of glucose (66-73). This metabolic signaling is purportedly required for the infarct-sparing effect of ischemic preconditioning (66-70), and has been implicated to play a secondary role in postconditioning (74).
Finally, there are intriguing but as-yet largely unexplored mediators that (I) have been reported to play a role in myocardial I/R injury; and (II) appear to have mechanistic links with both hyperglycemia/diabetes and ischemic conditioning. For example, p66Shc is a pro-oxidant protein, reportedly associated with the mPTP, which has been proposed to serve as a nexus for the deleterious effects of hyperglycemia and obesity-induced impairment in molecular signaling in multiple cell types including cardiomyocytes (75-77). Moreover, there is evidence that genetic knockout of p66Shc renders the heart resistant to infarction, possibly via attenuation of mitochondrial ROS production (78). A second molecular strategy that appears to mimic the favorable effects of ischemic conditioning—and, indeed, has been implicated to contribute to the infarct-sparing effect of remote perconditioning—is inhibition of arginase 2 signaling and the accompanying increase in nitric oxide production, activation of PKCε and targeting of the mitochondrial KATP channel (79-81). Interestingly, recent data have revealed that remote perconditioning fails to inhibit arginase 2 signaling in a rat model of type-1 diabetes (81). Whether a similar response is seen in the setting of type-2 diabetes is, at present, unknown.
Cardiac consequences of diabetes
The big picture: diabetes, CVD and AMI
The successful clinical translation of novel interventions to attenuate myocardial I/R injury has been identified as a major unmet need (8-10). This issue is of particular relevance and importance to patients with type-2 diabetes, as underscored by: (I) the ≥2-fold greater incidence of CVD, acute coronary syndromes and AMI in this patient cohort; (II) evidence of larger infarct sizes and exacerbated necrotic and apoptotic cell death; and thus, perhaps not surprisingly; (III) a 2- to 4-fold greater incidence of CVD-related mortality in diabetic versus non-diabetic subjects (1,82-93). Indeed, CVD is the leading cause of death and disability among diabetics, a poor prognosis that has not been appreciably influenced by the current trend of an overall reduction in mortality associated with AMI (1,16,82,83,92,93). Recent statistics reveal that, at present, Americans have an estimated ~40% lifetime risk for the development of diagnosed diabetes, and a sustained increase in the incidence of type-2 diabetes in the USA and worldwide is predicted for the next 20-30 years. This anticipated escalation in the numbers of patients with diabetes may have the potential to diminish the gains that have been made attenuating the overall prevalence of CVD-related death (1,94-96).
The cellular/molecular perspective
The hallmarks of type-2 diabetes are insulin resistance and accompanying metabolic dysregulation. Reduced insulin sensitivity has both direct effects on glucose uptake and insulin-mediated signaling in cardiac cells, and indirect cellular effects that are secondary to the accompanying hyperglycemia, hyperinsulinemia and hyperlipidemia (97-100). Most importantly in terms of cardioprotection, type-2 diabetes has been associated with impaired PI3 kinase/Akt signaling (components of both insulin signaling and the RISK pathway) and GLUT4 protein expression and/or translocation, as well as defects in AMPK and, indeed, essentially all kinases proposed to contribute to the infarct-sparing effect of ischemic conditioning. For example, impaired phosphorylation of PKC, PI3 kinase/Akt, ERK, STAT3, and GSK-3β has been described in diabetic hearts, possibly due to reported increases in activities of multiple phosphatases including phosphatase and tensin homolog (PTEN), MAPK phosphatases (MKPs) and protein phosphatase-2C (PP2C). There is evidence to suggest that downstream targets and proposed end-effectors of conditioning-induced cardioprotection are also modified by type-2 diabetes, including alterations in expression and activity of mitochondrial KATP channels and increased propensity of mPTP opening in response to increased intracellular Ca2+ concentrations in the diabetic myocardium (100-106). Although these insights have, not surprisingly, been largely derived from genetic rodent models of type-2 diabetes, including db/db and ob/ob mice and strains of fatty and lean rats (Zucker fatty, Otsuka Long-Evans-Tokushima fatty and Goto-Kakizaki) (100-104), analysis of myocardial tissue samples collected from diabetic patients at the time of cardiac surgery have yielded corroborative results (105,106).
Diabetes, infarct size and ischemic conditioning
Diabetes and I/R injury
The effect of type-2 diabetes on the infarct-sparing effect of ischemic conditioning has, almost exclusively, been assessed in the aforementioned rodent models. Accordingly, meaningful discussion of conditioning-induced cardioprotection in these models first requires an understanding of the effect of type-2 diabetes on infarct size in untreated control animals.
In contrast to the clinical consensus that diabetes is associated with larger infarct sizes and poor outcomes when compared with non-diabetic patients (1,82-93), data obtained in preclinical models are mixed: diabetes has been reported to increase, decrease or have no effect on cardiomyocyte death (19,107-109). These disparate outcomes have been attributed to multiple factors, including: (I) the duration of the diabetic state at the time of experimentation (with short-term diabetes more typically associated with an apparent reduced sensitivity to I/R injury); (II) differences over time or among models in levels of insulin and fatty acids; and (III) the presence or absence of obesity (19,108,109). The definitions of ‘short-term’ diabetes, hyperinsulinemia, hyperlipidemia, etc., and precise relationships of these factors with myocardial infarct size, are nebulous. However, it is important to emphasize: the translational relevance of experimental models characterized by a reduced sensitivity of the diabetic heart to I/R injury is considered to be questionable (108).
Ischemic conditioning in models of type-2 diabetes: is efficacy maintained?
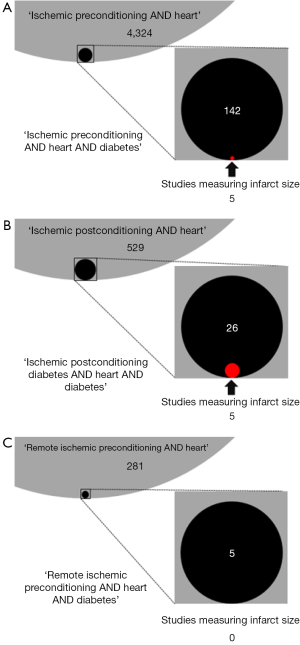
A recent (September 2014) PubMed search retrieved >5,000 papers focused on ischemic pre-, post- and remote conditioning in heart (Figure 3). Remarkably, addition of the term ‘diabetes’ to each search yielded only 173 papers (3.4% of the total number of publications) and, among these, only 10 included myocardial infarct size (the ‘gold standard’ of conditioning-induced cardioprotection) as one of the endpoints (Figure 3). The fact that <0.2% of currently published studies have specifically investigated the infarct-sparing effect of ischemic conditioning in of type-2 diabetes is extraordinary given the well-documented, profound consequences of diabetes on cardiac pathophysiology.
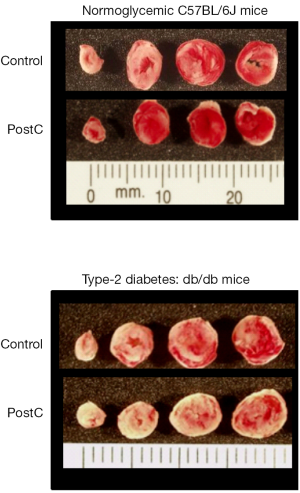
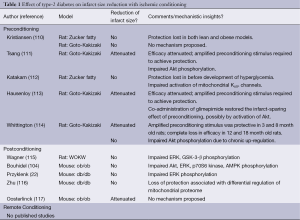
Among the small number of studies that have addressed this issue, a spectrum of rat and mouse models of type-2 diabetes have been utilized (Table 1). Nonetheless, irrespective of the model used and variations in study design, there is an emerging consensus: both pre- and post-conditioning either fail to reduce infarct size (Figure 2) (22,104,110,112,114-116), or the efficacy of conditioning is attenuated such that an amplified stimulus (i.e., an increased number of episodes of preconditioning ischemia) is required to evoke protection (111,113,117). Loss of conditioning-induced cardioprotection has been described in both lean and obese type-2 diabetic rat models (110) and in obese rats before the onset of significant hyperglycemia (112). Moreover, similar outcomes (a diminished responsiveness to the infarct-sparing effect of ischemic conditioning) have also been reported in models of type-1 diabetes (14,17-19,22,69,79-81,118-126), suggesting that the refractoriness of the diabetic heart to ischemic conditioning is not a simple consequence of hyperglycemia, hyperinsulinemia, hyperlipidemia, obesity or, in all likelihood, any single pathophysiological feature of the disease. Nascent mechanistic insight has, however, been obtained into the cellular mechanisms that may contribute to the compromised efficacy of ischemic conditioning in diabetic models. Perhaps not surprisingly, the complete or partial failure of pre- and postconditioning to limit infarct size in models of type-2 (and type-1) diabetes has largely been attributed to defects in RISK and AMPK signaling, with desensitization or impaired activation of multiple kinases (including PI3 kinase/Akt, ERK, p70S6 kinase, and/or GSK-3β), possibly due to augmented activities of MKPs and other phosphatases, all having been implicated to play a role (Table 1) (14,18,19,22,104,111,114,115,124). Potential diabetes-associated defects in mitochondrial end-effectors have also been identified, including impaired activation of mitochondrial KATP channels (112,116).
Full table
Finally, there is a notable and fundamental gap in our current knowledge of the efficacy of ischemic conditioning in models of type-2 diabetes. All previous discussion has focused exclusively on pre- and postconditioning; to date, no published studies have utilized remote conditioning as the cardioprotective trigger (Table 1). There is, however, one piece of evidence that diabetes may have a complex, confounding effect on the production or release of the as-yet unidentified humoral factor(s) from the site of the conditioning stimulus (127). A model of ‘transferred’ protection was used, in which the conditioning stimulus (brief repeated episodes of limb ischemia) was applied to diabetic and non-diabetic patient cohorts, serum was collected, dialyzed and administered to a remote target (isolated buffer-perfused rabbit hearts), and the hearts were then subjected to a sustained period of ischemia. For both cohorts, serum collected after the conditioning stimulus rendered the rabbit hearts resistant to infarction: i.e., type-2 diabetes per se did not preclude the infarct-sparing effect of remote conditioning. However, in the subset of diabetic subjects with peripheral neuropathy, the transferred serum failed to reduce infarct size in acceptor rabbit hearts, implicating the requisite involvement of a diabetes-sensitive neurogenic component in this model of humorally-mediated remote conditioning (127). The consequences of type-2 diabetes in standard in vivo models of remote conditioning, and the concept that persistent efficacy of remote conditioning in diabetic models may depend on the intact innervation of the effector organ, are topics of ongoing study by our group and others.
Initial insight: ischemic conditioning in diabetic patients
The wealth of preclinical evidence documenting reduction of infarct size with pre-, post- and remote conditioning has provided the groundwork and rationale for ongoing efforts to translate the concept of endogenous conditioning-induced cardioprotection for the clinical treatment of myocardial I/R injury (6,8-10,128). As preconditioning is, by definition, a pretreatment—thereby limiting its potential for clinical use to planned ischemic events such as cardiac surgery and elective percutaneous intervention (PCI)—current attention is focused largely on postconditioning and remote conditioning. Results from ~40 phase II clinical trials have been reported, and large phase III trials are in progress [reviewed in (6,128)]. Overall, the data have been mixed: ~60% of the studies observed significant reductions in cardiac enzyme release and other surrogate endpoints reflecting myocardial infarct size in conditioned cohorts versus controls, while the remainder reported either no effect or exacerbated outcomes (6,128). In addition, recent meta-analyses of pooled data from multiple trials underscored the variability among studies and concluded that, at present, there is borderline evidence, or no evidence, for cardioprotection with either postconditioning or remote conditioning (129,130).
In addition to differences in patient demographics and enrollment criteria, protocol logistics (including the number and timing of the conditioning stimuli and duration of sustained ischemia), choice of endpoints, etc., extrapolation of the results obtained in preclinical models of type-2 diabetes suggest that two related factors—the confounding effects of diabetes, together with differing proportions of diabetic patients among studies—may also contribute to the aforementioned variability. Indeed, in an effort to mitigate this concern, some investigators have prospectively excluded the enrollment of diabetic patients (131-134). Initial evidence appears to support of the concept that conditioning-induced cardioprotection may be impaired or lost in patients with diabetes. For example, in two clinical trials in which prospective subset analyses were performed and cardiac enzyme release served as the surrogate for infarct size, preconditioning (triggered by prodromal angina) had no beneficial effect, while postconditioning tended to exacerbate myocardial injury in diabetic cohorts (135,136). In addition, in a third trial evaluating the efficacy of remote conditioning administered following elective PCI, the incidence of post-procedural MI was significantly increased in patients with versus without diabetes (137). Finally, in an ex vivo analysis, preconditioning attenuated hypoxia-reoxygenation-induced cell death in human atrial samples harvested from non-diabetic patients at the time of cardiac surgery, but failed to render atrial tissue resistant to injury in samples obtained from patients with type-2 diabetes (138).
Summary and future directions
Despite the paucity of studies conducted in preclinical models of type-2 diabetes in which myocardial infarct size was among the primary endpoints (Table 1 and Figure 3), a consensus is emerging: the diabetic rodent heart is refractory to the profound infarct-sparing effect of preconditioning, postconditioning and, possibly, remote conditioning. It could be argued these data may be of limited clinical relevance, given the overt simplicity of the mouse and rat models: the duration of diabetes is comparatively acute (on the order of weeks, rather than months-years), and, with few exceptions (22,113,114), the models (I) do not mimic the multiple comorbid conditions seen in substantial subsets of patients; and (II) do not include groups treated with the battery of pharmacologic agents that would be administered to patients as standard clinical care for both AMI and the management of hyperglycemia. Nonetheless, although far from conclusive, the initial clinical data appear to corroborate the preclinical results.
Does ischemic conditioning have the potential to achieve the as-yet unmet clinical challenge of attenuating myocardial I/R injury, reducing infarct size and improving outcome in patients post-MI? And, if so: is the efficacy of conditioning-induced cardioprotection compromised in diabetic patients? Definitive resolution of these issues awaits the completion of Phase III clinical trials that are prospectively designed with sufficient statistical power to discern the presence versus absence of an infarct-sparing effect of ischemic conditioning in stratified subgroups of patients with and without type-2 diabetes.
Acknowledgements
Authors’ contributions: JW conducted a critical review of the literature, prepared the first draft of the manuscript and proof-read/approved the final version. KP provided assistance and confirmed the accuracy of the literature review, edited the manuscript and proof-read the final version.
Disclosure: KP serves on the Scientific Advisory Board of Infarct Reduction Technologies, Inc. The authors declare no conflict of interest.
References
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28-e292. [PubMed]
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713-9. [PubMed]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121-35. [PubMed]
- Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 2011;301:H1723-41. [PubMed]
- Przyklenk K. Reduction of myocardial infarct size with ischemic “conditioning”: physiologic and technical considerations. Anesth Analg 2013;117:891-901. [PubMed]
- Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet 2013;381:166-75. [PubMed]
- Thompson PL, Fletcher EE, Katavatis V. Enzymatic indices of myocardial necrosis: influence on short- and long-term prognosis after myocardial infarction. Circulation 1979;59:113-9. [PubMed]
- Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther 2011;16:332-9. [PubMed]
- Schwartz Longacre L, Kloner RA, Arai AE, et al. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation 2011;124:1172-9. [PubMed]
- Hausenloy DJ, Erik Botker H, Condorelli G, et al. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2013;98:7-27. [PubMed]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124-36. [PubMed]
- Zhao ZQ, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 2003;285:H579-88. [PubMed]
- Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893-9. [PubMed]
- Przyklenk K. Efficacy of cardioprotective ‘conditioning’ strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging. 2011;28:331-43. [PubMed]
- Selvin E, Parrinello CM, Sacks DB, et al. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517-25. [PubMed]
- Beckman JA, Paneni F, Cosentino F, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J 2013;34:2444-52. [PubMed]
- Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 2007;59:418-58. [PubMed]
- Vinten-Johansen J, Granfeldt A, Mykytenko J, et al. The multidimensional physiological responses to postconditioning. Antioxid Redox Signal 2011;14:791-810. [PubMed]
- Miki T, Itoh T, Sunaga D, et al. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol 2012;11:67. [PubMed]
- Ferdinandy P, Hausenloy DJ, Heusch G, et al. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142-74. [PubMed]
- Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002;106:2881-3. [PubMed]
- Przyklenk K, Maynard M, Greiner DL, et al. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal 2011;14:781-90. [PubMed]
- Przyklenk K, Kloner RA. Ischemic preconditioning: exploring the paradox. Prog Cardiovasc Dis 1998;40:517-47. [PubMed]
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 2003;83:1113-51. [PubMed]
- Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 2008;88:581-609. [PubMed]
- Cohen MV, Downey JM. Is it time to translate ischemic preconditioning’s mechanism of cardioprotection into clinical practice? J Cardiovasc Pharmacol Ther 2011;16:273-80. [PubMed]
- Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915-9. [PubMed]
- Liu GS, Thornton J, Van Winkle DM, et al. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation 1991;84:350-6. [PubMed]
- Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol 2008;103:203-15. [PubMed]
- Goto M, Liu Y, Yang XM, et al. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res 1995;77:611-21. [PubMed]
- Schultz JE, Hsu AK, Gross GJ. Ischemic preconditioning in the intact rat heart is mediated by delta1- but not mu- or kappa-opioid receptors. Circulation 1998;97:1282-9. [PubMed]
- Cohen MV, Yang XM, Liu GS, et al. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial K(ATP) channels. Circ Res 2001;89:273-8. [PubMed]
- Lecour S, Smith RM, Woodward B, et al. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol 2002;34:509-18. [PubMed]
- Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol 1994;266:H1145-52. [PubMed]
- Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 2004;61:448-60. [PubMed]
- Lacerda L, Somers S, Opie LH, et al. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res 2009;84:201-8. [PubMed]
- Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther 2001;89:29-46. [PubMed]
- Hausenloy DJ, Maddock HL, Baxter GF, et al. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res 2002;55:534-43. [PubMed]
- Ruiz-Meana M, Rodriguez-Sinovas A, Cabestrero A, et al. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia-reperfusion injury. Cardiovasc Res 2008;77:325-33. [PubMed]
- Murphy E, Steenbergen C. What makes the mitochondria a killer? Can we condition them to be less destructive? Biochim Biophys Acta 2011;1813:1302-8.
- Argaud L, Gateau-Roesch O, Raisky O, et al. Postconditioning inhibits mitochondrial permeability transition. Circulation 2005;111:194-7. [PubMed]
- Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal 2011;14:893-907. [PubMed]
- Vinten-Johansen J, Shi W. Perconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directions. J Cardiovasc Pharmacol Ther 2011;16:260-6. [PubMed]
- Przyklenk K, Whittaker P. Remote ischemic preconditioning: current knowledge, unresolved questions, and future priorities. J Cardiovasc Pharmacol Ther 2011;16:255-9. [PubMed]
- Przyklenk K, Whittaker P. Genesis of remote conditioning: action at a distance—‘hypotheses non fingo’? J Cardiovasc Med (Hagerstown) 2013;14:180-6. [PubMed]
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 2008;79:377-86. [PubMed]
- Saxena P, Newman MA, Shehatha JS, et al. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg 2010;25:127-34. [PubMed]
- Dickson EW, Blehar DJ, Carraway RE, et al. Naloxone blocks transferred preconditioning in isolated rabbit hearts. J Mol Cell Cardiol 2001;33:1751-6. [PubMed]
- Serejo FC, Rodrigues LF Jr, da Silva Tavares KC, et al. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol 2007;49:214-20. [PubMed]
- Shimizu M, Tropak M, Diaz RJ, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191-200. [PubMed]
- Donato M, Buchholz B, Rodriguez M, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol 2013;98:425-34. [PubMed]
- Gourine A, Gourine AV. Neural mechanisms of cardioprotection. Physiology (Bethesda) 2014;29:133-40. [PubMed]
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 2010;105:651-5. [PubMed]
- Redington KL, Disenhouse T, Strantzas SC, et al. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol 2012;107:241. [PubMed]
- Bertolotto C, Maulon L, Filippa N, et al. Protein kinase C theta and epsilon promote T-cell survival by a rsk-dependent phosphorylation and inactivation of BAD. J Biol Chem 2000;275:37246-50. [PubMed]
- Penna C, Rastaldo R, Mancardi D, et al. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol 2006;101:180-9. [PubMed]
- Philipp S, Yang XM, Cui L, et al. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res 2006;70:308-14. [PubMed]
- Weinbrenner C, Nelles M, Herzog N, et al. Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: a newly identified non-neuronal but PKC-dependent pathway. Cardiovasc Res 2002;55:590-601. [PubMed]
- Simkhovich BZ, Przyklenk K, Kloner RA. Role of protein kinase C in ischemic “conditioning”: from first evidence to current perspectives. J Cardiovasc Pharmacol Ther 2013;18:525-32. [PubMed]
- Skyschally A, Schulz R, Gres P, et al. Attenuation of ischemic preconditioning in pigs by scavenging of free oxyradicals with ascorbic acid. Am J Physiol Heart Circ Physiol 2003;284:H698-703. [PubMed]
- Hausenloy D, Wynne A, Duchen M, et al. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation 2004;109:1714-7. [PubMed]
- Jennings RB. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res 2013;113:428-38. [PubMed]
- Reimer KA, Murry CE, Yamasawa I, et al. Four brief periods of myocardial ischemia cause no cumulative ATP loss or necrosis. Am J Physiol 1986;251:H1306-15. [PubMed]
- Murry CE, Richard VJ, Reimer KA, et al. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res 1990;66:913-31. [PubMed]
- Murry CE, Richard VJ, Jennings RB, et al. Myocardial protection is lost before contractile function recovers from ischemic preconditioning. Am J Physiol 1991;260:H796-804. [PubMed]
- Yoshiyama M, Merkle H, Garwood M, et al. Transmural distribution of 2-deoxyglucose uptake in normal and post-ischemic canine myocardium. NMR Biomed 1995;8:9-18. [PubMed]
- Tong H, Chen W, London RE, et al. Preconditioning enhanced glucose uptake is mediated by p38 MAP kinase not by phosphatidylinositol 3-kinase. J Biol Chem 2000;275:11981-6. [PubMed]
- Yabe K, Tanonaka K, Koshimizu M, et al. A role of PKC in the improvement of energy metabolism in preconditioned heart. Basic Res Cardiol 2000;95:215-27. [PubMed]
- Ji L, Zhang X, Liu W, et al. AMPK-regulated and Akt-dependent enhancement of glucose uptake is essential in ischemic preconditioning-alleviated reperfusion injury. PLoS One 2013;8:e69910. [PubMed]
- Nederlof R, Eerbeek O, Hollmann MW, et al. Targeting hexokinase II to mitochondria to modulate energy metabolism and reduce ischaemia-reperfusion injury in heart. Br J Pharmacol 2014;171:2067-79. [PubMed]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007;8:774-85. [PubMed]
- Gürel E, Smeele KM, Eerbeek O, et al. Ischemic preconditioning affects hexokinase activity and HKII in different subcellular compartments throughout cardiac ischemia-reperfusion. J Appl Physiol (1985) 2009;106:1909-16. [PubMed]
- Pasdois P, Parker JE, Halestrap AP. Extent of mitochondrial hexokinase II dissociation during ischemia correlates with mitochondrial cytochrome c release, reactive oxygen species production, and infarct size on reperfusion. J Am Heart Assoc 2013;2:e005645. [PubMed]
- Hermann R, Marina Prendes MG, Torresin ME, et al. Effects of the AMP-activated protein kinase inhibitor compound C on the postconditioned rat heart. J Physiol Sci 2012;62:333-41. [PubMed]
- Di Lisa F, Kaludercic N, Carpi A, et al. Mitochondrial pathways for ROS formation and myocardial injury: the relevance of p66(Shc) and monoamine oxidase. Basic Res Cardiol 2009;104:131-9. [PubMed]
- Paneni F, Costantino S, Cosentino F. p66(Shc)-induced redox changes drive endothelial insulin resistance. Atherosclerosis 2014;236:426-9. [PubMed]
- Savino C, Pelicci P, Giorgio M. The P66Shc/mitochondrial permeability transition pore pathway determines neurodegeneration. Oxid Med Cell Longev 2013;2013:719407.
- Carpi A, Menabò R, Kaludercic N, et al. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta 2009;1787:774-80.
- Gonon AT, Jung C, Katz A, et al. Local arginase inhibition during early reperfusion mediates cardioprotection via increased nitric oxide production. PLoS One 2012;7:e42038. [PubMed]
- Tratsiakovich Y, Gonon AT, Krook A, et al. Arginase inhibition reduces infarct size via nitric oxide, protein kinase C epsilon and mitochondrial ATP-dependent K+ channels. Eur J Pharmacol 2013;712:16-21. [PubMed]
- Kiss A, Tratsiakovich Y, Gonon AT, et al. The role of arginase and rho kinase in cardioprotection from remote ischemic perconditioning in non-diabetic and diabetic rat in vivo. PLoS One 2014;9:e104731. [PubMed]
- Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229-34. [PubMed]
- Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-22. [PubMed]
- Krempf M, Parhofer KG, Steg PG, et al. Cardiovascular event rates in diabetic and nondiabetic individuals with and without established atherothrombosis (from the REduction of Atherothrombosis for Continued Health [REACH] Registry). Am J Cardiol 2010;105:667-71. [PubMed]
- Alegria JR, Miller TD, Gibbons RJ, et al. Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am Heart J 2007;154:743-50. [PubMed]
- Marso SP, Miller T, Rutherford BD, et al. Comparison of myocardial reperfusion in patients undergoing percutaneous coronary intervention in ST-segment elevation acute myocardial infarction with versus without diabetes mellitus (from the EMERALD Trial). Am J Cardiol 2007;100:206-10. [PubMed]
- Mukamal KJ, Nesto RW, Cohen MC, et al. Impact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarction. Diabetes Care 2001;24:1422-7. [PubMed]
- Murcia AM, Hennekens CH, Lamas GA, et al. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med 2004;164:2273-9. [PubMed]
- Zia MI, Ghugre NR, Roifman I, et al. Comparison of the frequencies of myocardial edema determined by cardiac magnetic resonance in diabetic versus nondiabetic patients having percutaneous coronary intervention for ST elevation myocardial infarction. Am J Cardiol 2014;113:607-12. [PubMed]
- Chowdhry MF, Vohra HA, Galiñanes M. Diabetes increases apoptosis and necrosis in both ischemic and nonischemic human myocardium: role of caspases and poly-adenosine diphosphate-ribose polymerase. J Thorac Cardiovasc Surg 2007;134:124-31.e3.
- Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res 2000;87:1123-32. [PubMed]
- Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA 1999;281:1291-7. [PubMed]
- Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007;298:765-75. [PubMed]
- Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311-21. [PubMed]
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047-53. [PubMed]
- Gregg EW, Zhuo X, Cheng YJ, et al. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985-2011: a modelling study. Lancet Diabetes Endocrinol 2014. [Epub ahead of print].
- Abel ED, O’Shea KM, Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol 2012;32:2068-76. [PubMed]
- Hafstad AD, Solevag GH, Severson DL, et al. Perfused hearts from Type 2 diabetic (db/db) mice show metabolic responsiveness to insulin. Am J Physiol Heart Circ Physiol 2006;290:H1763-9. [PubMed]
- Carley AN, Atkinson LL, Bonen A, et al. Mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice. Arch Physiol Biochem 2007;113:65-75. [PubMed]
- Desrois M, Sidell RJ, Gauguier D, et al. Initial steps of insulin signaling and glucose transport are defective in the type 2 diabetic rat heart. Cardiovasc Res 2004;61:288-96. [PubMed]
- Huisamen B. Protein kinase B in the diabetic heart. Mol Cell Biochem 2003;249:31-8. [PubMed]
- Mocanu MM, Field DC, Yellon DM. A potential role for PTEN in the diabetic heart. Cardiovasc Drugs Ther 2006;20:319-21. [PubMed]
- Miki T, Miura T, Hotta H, et al. Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho-glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeability transition. Diabetes 2009;58:2863-72. [PubMed]
- Bouhidel O, Pons S, Souktani R, et al. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol 2008;295:H1580-6. [PubMed]
- Wang B, Raedschelders K, Shravah J, et al. Differences in myocardial PTEN expression and Akt signalling in type 2 diabetic and nondiabetic patients undergoing coronary bypass surgery. Clin Endocrinol (Oxf) 2011;74:705-13. [PubMed]
- Anderson EJ, Rodriguez E, Anderson CA, et al. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am J Physiol Heart Circ Physiol 2011;300:H118-24. [PubMed]
- Liu Y, Thornton JD, Cohen MV, et al. Streptozotocin-induced non-insulin-dependent diabetes protects the heart from infarction. Circulation 1993;88:1273-8. [PubMed]
- Paulson DJ. The diabetic heart is more sensitive to ischemic injury. Cardiovasc Res 1997;34:104-12. [PubMed]
- Balakumar P, Sharma NK. Healing the diabetic heart: does myocardial preconditioning work? Cell Signal 2012;24:53-9. [PubMed]
- Kristiansen SB, Lofgren B, Stottrup NB, et al. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia 2004;47:1716-21. [PubMed]
- Tsang A, Hausenloy DJ, Mocanu MM, et al. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes 2005;54:2360-4. [PubMed]
- Katakam PV, Jordan JE, Snipes JA, et al. Myocardial preconditioning against ischemia-reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am J Physiol Regul Integr Comp Physiol 2007;292:R920-6. [PubMed]
- Hausenloy DJ, Wynne AM, Mocanu MM, et al. Glimepiride treatment facilitates ischemic preconditioning in the diabetic heart. J Cardiovasc Pharmacol Ther 2013;18:263-9. [PubMed]
- Whittington HJ, Harding I, Stephenson CI, et al. Cardioprotection in the aging, diabetic heart: the loss of protective Akt signalling. Cardiovasc Res 2013;99:694-704. [PubMed]
- Wagner C, Kloeting I, Strasser RH, et al. Cardioprotection by postconditioning is lost in WOKW rats with metabolic syndrome: role of glycogen synthase kinase 3beta. J Cardiovasc Pharmacol 2008;52:430-7. [PubMed]
- Zhu SG, Xi L, Kukreja RC. Type 2 diabetic obese db/db mice are refractory to myocardial ischaemic post-conditioning in vivo: potential role for Hsp20, F1-ATPase delta and Echs1. J Cell Mol Med 2012;16:950-8. [PubMed]
- Oosterlinck W, Dresselaers T, Geldhof V, et al. Diabetes mellitus and the metabolic syndrome do not abolish, but might reduce, the cardioprotective effect of ischemic postconditioning. J Thorac Cardiovasc Surg 2013;145:1595-602. [PubMed]
- Kersten JR, Toller WG, Gross ER, et al. Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. Am J Physiol Heart Circ Physiol 2000;278:H1218-24. [PubMed]
- Nieszner E, Posa I, Kocsis E, et al. Influence of diabetic state and that of different sulfonylureas on the size of myocardial infarction with and without ischemic preconditioning in rabbits. Exp Clin Endocrinol Diabetes 2002;110:212-8. [PubMed]
- Yadav HN, Singh M, Sharma PL. Involvement of GSK-3beta in attenuation of the cardioprotective effect of ischemic preconditioning in diabetic rat heart. Mol Cell Biochem 2010;343:75-81. [PubMed]
- Drenger B, Ostrovsky IA, Barak M, et al. Diabetes blockade of sevoflurane postconditioning is not restored by insulin in the rat heart: phosphorylated signal transducer and activator of transcription 3- and phosphatidylinositol 3-kinase-mediated inhibition. Anesthesiology 2011;114:1364-72. [PubMed]
- Fan Y, Yang S, Zhang X, et al. Comparison of cardioprotective efficacy resulting from a combination of atorvastatin and ischaemic post-conditioning in diabetic and non-diabetic rats. Clin Exp Pharmacol Physiol 2012;39:938-43. [PubMed]
- Badalzadeh R, Mohammadi M, Najafi M, et al. The additive effects of ischemic postconditioning and cyclosporine-A on nitric oxide activity and functions of diabetic myocardium injured by ischemia/reperfusion. J Cardiovasc Pharmacol Ther 2012;17:181-9. [PubMed]
- Liu M, Zhou B, Xia ZY, et al. Hyperglycemia-induced inhibition of DJ-1 expression compromised the effectiveness of ischemic postconditioning cardioprotection in rats. Oxid Med Cell Longev 2013;2013:564902.
- Potier L, Waeckel L, Vincent MP, et al. Selective kinin receptor agonists as cardioprotective agents in myocardial ischemia and diabetes. J Pharmacol Exp Ther 2013;346:23-30. [PubMed]
- Han Z, Cao J, Song D, et al. Autophagy is involved in the cardioprotection effect of remote limb ischemic postconditioning on myocardial ischemia/reperfusion injury in normal mice, but not diabetic mice. PLoS One 2014;9:e86838. [PubMed]
- Jensen RV, Stottrup NB, Kristiansen SB, et al. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol 2012;107:285. [PubMed]
- Ovize M, Thibault H, Przyklenk K. Myocardial conditioning: opportunities for clinical translation. Circ Res 2013;113:439-50. [PubMed]
- Schevchuck A, Laskey WK. Ischemic conditioning as an adjunct to percutaneous coronary intervention. Circ Cardiovasc Interv 2013;6:484-92. [PubMed]
- Healy DA, Khan WA, Wong CS, et al. Remote preconditioning and major clinical complications following adult cardiovascular surgery: Systematic review and meta-analysis. Int J Cardiol 2014;176:20-31. [PubMed]
- Venugopal V, Hausenloy DJ, Ludman A, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart 2009;95:1567-71. [PubMed]
- Thielmann M, Kottenberg E, Boengler K, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 2010;105:657-64. [PubMed]
- Heusch G, Musiolik J, Kottenberg E, et al. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res 2012;110:111-5. [PubMed]
- Kottenberg E, Thielmann M, Bergmann L, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol - a clinical trial. Acta Anaesthesiol Scand 2012;56:30-8. [PubMed]
- Ishihara M, Inoue I, Kawagoe T, et al. Diabetes mellitus prevents ischemic preconditioning in patients with a first acute anterior wall myocardial infarction. J Am Coll Cardiol 2001;38:1007-11. [PubMed]
- Yetgin T, Magro M, Manintveld OC, et al. Impact of multiple balloon inflations during primary percutaneous coronary intervention on infarct size and long-term clinical outcomes in ST-segment elevation myocardial infarction: real-world postconditioning. Basic Res Cardiol 2014;109:403. [PubMed]
- Carrasco-Chinchilla F, Munoz-Garcia AJ, Dominguez-Franco A, et al. Remote ischaemic postconditioning: does it protect against ischaemic damage in percutaneous coronary revascularisation? Randomised placebo-controlled clinical trial. Heart 2013;99:1431-7. [PubMed]
- Hassouna A, Loubani M, Matata BM, et al. Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc Res 2006;69:450-8. [PubMed]