Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis
Introduction
Diabetes is an independent risk factor for cardiovascular disease (1). Adults with diabetes have a 2-4 times higher risk for heart disease or stroke, compared with those without diabetes (2).
Among Seventh-day Adventists, the prevalence of type 2 diabetes among individuals consuming vegetarian diets was observed to be approximately half of that in those following an omnivorous dietary pattern (3,4). Other cohort studies have also suggested that meat consumption is associated with an increased risk of type 2 diabetes (5,6). In clinical trials, glycemic control was found to improve following switch from an omnivorous diet to a vegetarian diet (7), suggesting that the latter may be useful in both the prevention and treatment of type 2 diabetes. However, many studies using vegetarian diets had short durations (e.g., <4 weeks) or small sample sizes, limiting the ability to identify effects of diet on type 2 diabetes. To our knowledge, no meta-analysis of studies on the association of vegetarian (ovo-lacto vegetarian or vegan) diets with hemoglobin A1c (HbA1c) and blood glucose levels has been conducted. To provide a valid estimate of the effect size regarding the benefits of consumption of vegetarian diets in patients with type 2 diabetes, which could be useful for dietary recommendations, we conducted a meta-analysis of studies to examine associations between vegetarian diets and glycemic control in type 2 diabetes. This investigation was registered on PROSPERO (registration number CRD42013004370) on April 26, 2013.
Methods
The study was conducted in accordance with PRISMA guidelines (8). The published scientific literature was searched for clinical trials in adults in which vegetarian diets (defined as those excluding meat, poultry, and fish) or vegan diets (defined as excluding animal-derived food products) were used as interventions for at least 4 weeks and changes in HbA1c and/or fasting blood glucose levels were reported. The primary measure of interest was the HbA1c level in patients consuming vegetarian or vegan diets, compared with levels in patients in the control group (omnivorous diet). A secondary endpoint was the change in the fasting blood glucose level.
Search strategy and article selection
Publications were identified by searches of PubMed (1946 through December 9, 2013), Excerpta Medica Database (EMBASE) (1947 through December 9, 2013), Web of Science (1900 through December 9, 2013), and Cochrane Central Register of Controlled Trials (1966 through December 9, 2013). The following criteria were used for inclusion in the meta-analysis: (I) age of participants >20 years; (II) vegetarian diet as intervention; (III) HbA1c or blood glucose levels as outcomes; (IV) controlled trials with durations of ≥4 weeks. Exclusion criteria were: (I) not an original investigation; (II) duplicate samples; (III) diabetes other than type 2; (IV) multiple interventions; and (V) uncontrolled studies. There were no restrictions regarding sex, race, ethnicity, language, sample size, publication status, or publication date.
The strategy used for the PubMed search is shown in Table 1. Analogous terms were used for the EMBASE, Web of Science, and Cochrane searches. Two researchers (YY and SML) independently reviewed the titles and abstracts of all citations retrieved. For citations appearing to meet the inclusion criteria, the same researchers independently reviewed the full-text articles to identify eligible studies. Disagreements were resolved by consensus. From the reference lists of reviewed articles and through contacts with research experts, additional articles were identified and reviewed for eligibility.
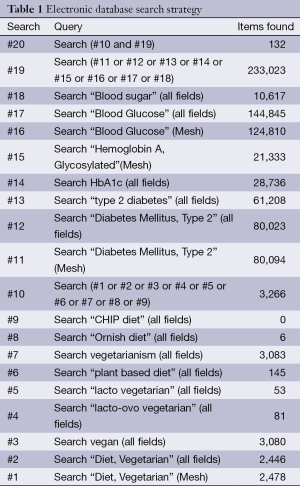
Full table
Data extraction
For each study, data were collected on the following: HbA1c and fasting blood glucose levels; study methodology and sample size; baseline characteristics of the study population, including mean age, sex (proportion of men), body mass index (BMI), and type of diabetes; and outcomes, including adjustment factors used for each analytic model. Mean values for baseline age, proportion of male subjects, BMI, and HbA1c and fasting blood glucose levels were calculated.
Quality measures
Using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (9), the quality of the studies included in the statistical analysis was assessed. These criteria included measures to minimize selection bias (random sequence generation and allocation concealment), performance, detection, attrition (incomplete outcome data), and reporting. Masking of dietary assignment was not used as a quality criterion, because it is impracticable in studies on prescribed diets.
Data synthesis and analysis
Mean differences in HbA1c and fasting blood glucose levels between patients assigned to receive the vegetarian and comparator diets were calculated. The pooled standard error (SE) for the net difference in the HbA1c and fasting blood glucose levels associated with the consumption of a vegetarian diet was obtained or, when not given, estimated using the method of Follmann et al. (10), assuming a correlation of 0.50 between the baseline and final HbA1c values (parallel design) or between HbA1c values during the intervention and control periods (cross-over design).
Estimates of net changes in HbA1c levels, fasting blood glucose levels, energy, and selected dietary components (energy, carbohydrate, protein, fat, cholesterol, and fiber) associated with the consumption of vegetarian diets were combined using a random effects model, which assigns a weight to each study on the basis of the study’s inverse variance. Overall estimates were derived separately for controlled trials using the study as the unit of analysis. Estimates of differences in HbA1c levels were reported with 95% confidence intervals (CIs). Two-sided P values <0.05 were considered statistically significant.
Sensitivity analysis was carried out by assessing the effects of removal of each study on the combined effect. Calculation of I2 was performed with subgroups using the study as the unit of analysis to assess heterogeneity among studies (11).
Funnel plots were developed to identify publication bias, and Egger’s test (12) was performed to assess the relationship between sample size and effect size. The “trim and fill” method (13) was used to adjust for publication bias. This method determines where missing studies are likely to fall, adds them to the analysis, and then re-computes the combined effect (13). All analyses were performed using Comprehensive Meta-Analysis, version 2 software (Biostat, Englewood, NJ, USA) (14).
Results
Results of literature search
The PubMed search yielded 132 citations; those of the Web of Science, EMBASE, and the Cochrane Central Register of Controlled Trials retrieved 95, 229 and 35 publications, respectively (Figure 1). After removing duplicates, 477 unique publications were identified. Of these, six met the inclusion criteria (15-20). These had been carried out in Brazil, the Czech Republic, and the United States, and included six intervention groups using lacto-ovo vegetarian or vegan diets with a total of 255 participants (17 lacto-ovo vegetarian and 238 vegan) (Table 2). All studies included patients with type 2 diabetes.
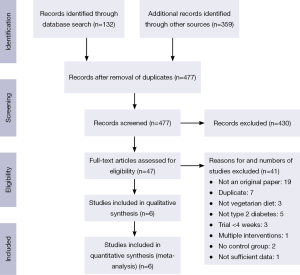
Full table
All were open (non-masked) controlled trials lasting for ≥4 weeks (mean duration 23.7 weeks; range, 4-74 weeks). Vegan diets were examined in five studies (15,17-20), and a lacto-ovo vegetarian diet in one (16). Five studies used parallel designs, (15,17-20) and one used a cross-over design (16). The mean age of participants was 52.5 years (range, 44.4-59.0 years).
Quality of studies
Of the six studies included, three were randomized, two were nonrandomized comparisons at two separate corporate sites (17), and one was a cluster randomized trial (19). Allocation concealment was not reported in the randomized trials and was not relevant to the remaining two trials. Because these studies were dietary intervention trials, none included blinding of participants. The outcome of interest was the objective measure of HbA1c level, and thus blinding of outcome assessment (detection bias) was unlikely to be an issue.
Pooled effects of vegetarian diets on HbA1c and fasting blood glucose levels
In the pooled analysis, consumption of vegetarian diets was associated with a significant mean reduction in HbA1c (–0.39 percentage point; 95% CI, –0.62 to –0.15; P=0.001; I2=3.0; P for heterogeneity =0.389), compared with omnivorous diets. The corresponding mean reduction in fasting blood glucose levels was not significant (–0.36 mmol/L; 95% CI, –1.04 to 0.32; P=0.301; I2=0; P for heterogeneity =0.710) (Figures 2,3).


Removal of one study at a time from the analysis did not substantially change the results, with significant differences in HbA1c levels between vegetarian and comparison groups ranging from –0.31 to –0.53 percentage point (P<0.05 for all).
Pooled effects of vegetarian diets on nutrient intakes
In the pooled analysis, consumption of vegetarian diets was associated with significant mean differences in energy intake (–139.8 kcal; 95% CI, –232.8 to –46.7; P=0.003; I2=0; P for heterogeneity =0.437), carbohydrate (13.8% energy; 95% CI, 3.7 to 23.9; P=0.008; I2=93.9; P for heterogeneity <0.001), protein (–6.4% energy; 95% CI, –9.8 to –3.0; P<0.001; I2=90.2; P for heterogeneity <0.001), total fat (−11.6% energy; 95% CI, −17.6 to −5.5; P<0.001, I2=93.2, P for heterogeneity <0.001), cholesterol (−172.5 mg; 95% CI, −221.3 to −123.6; P<0.001; I2=71.9; P for heterogeneity =0.003) and fiber (7.0 g; 95% CI, 4.2 to 9.7; P<0.001; I2=56.9; P for heterogeneity =0.041) (Table 3).
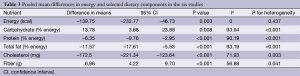
Full table
Publication bias
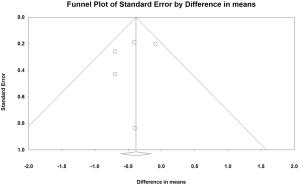
The funnel plot revealed that the smaller trials reporting reductions in HbA1c levels were possibly overrepresented (Figure 4). In the absence of publication bias, the study results would symmetrically represent the mean effect size; these findings suggested that some studies were missing in the bottom right side. However, this visual impression was not confirmed by Egger’s test (P=0.554). The results of the trim-and-fill method, which must be interpreted with substantial caution, suggested that two trials may have been missing and that their addition would change the overall effect on HbA1c to −0.26 percentage point (95% CI, −0.53 to 0.003).
Discussion
This meta-analysis revealed that a vegetarian dietary pattern significantly reduced HbA1c by 0.4 percentage point in patients with type 2 diabetes. The magnitude of the effect size is approximately one-half of that seen with metformin, which is used as first-line oral therapy for elevated HbA1c levels (21). A previous meta-analysis of nine randomized controlled trials found that the weighted mean difference in HbA1c levels between metformin and placebo was –0.9 percentage point (95% CI, –1.1 to –0.7) (22).
While vegetarian diets significantly improved HbA1c levels, the reductions seen in fasting blood glucose level did not reach statistical significance. Given that glucose levels are often variable and that baseline HbA1c level is a stronger predictor of subsequent diabetes and cardiovascular events than fasting glucose (23), these results still substantiate the beneficial effects of vegetarian diets in treatment of type 2 diabetes.
Vegetarian (including vegan) diets have benefits for cardiovascular health (24), hypertension (25), body weight (26), and plasma lipids (27), and also provide nutritional advantages compared with omnivorous diets (28). The evidence presented herein demonstrates that these diets also reduce HbA1c. These findings are consistent with those reported in a previous review of observational and clinical studies, namely that vegetarian diets improve glycemic control (7). Moreover, clinical data have also indicated that regular consumption of meat is a risk factor for type 2 diabetes (29,30). These results are in agreement with observations from Adventist Health Study 2. This large cohort study reported that the odds ratio for developing diabetes compared with non-vegetarians was 0.38 (95% CI, 0.24 to 0.62) for vegans and 0.62 (95% CI, 0.50 to 0.76) for lacto-ovo vegetarians (3,4).
To provide insight into the possible mechanisms associated with the HbA1c-lowering effect of a vegetarian diet, we extended this meta-analysis to analyze changes in energy and dietary components due to vegetarian diet interventions. Importantly, decreases in energy (−139.8 kcal), protein (−6.4% energy), total fat (−11.6% energy), and cholesterol intakes (−172.5 mg) were observed, along with increases in carbohydrate (13.8% energy) and fiber intakes (7.0 g). These observations suggest several possible mechanisms that may explain the effect of a vegetarian diet on improved glycemic control. First, a reduction in energy intake is likely to be associated with weight loss, which is known to improve glycemic control (31,32). A recent review indicated that even in the absence of specific limits on energy intake or portion sizes, vegetarian diets are associated with lower body weight compared to an untreated control group (26). However, weight loss may not be the only factor responsible for the HbA1c-lowering effects of vegetarian diets. In a study by Anderson and Ward, a low-fat, high-carbohydrate diet was associated with improved glycemic control in the absence of weight loss (33).
Intramyocellular lipid accumulation is strongly associated with insulin resistance (34,35). High-fat diets appear to downregulate the expression of genes needed for mitochondrial oxidative phosphorylation in skeletal muscle, thereby contributing to an increase in intramyocellular lipid concentration (36). Switching to a vegetarian diet provides roughly 10% less fat (as a percentage of total energy intake), according to our analysis, which would be expected to reduce the concentration of intramyocellular lipid. In a case-control study, intramyocellular lipid concentrations in soleus muscle were approximately 30% lower in a group of 21 vegans than those in 25 individuals on an omnivorous diet matched for sex, age, and body weight (P=0.01) (37). Also, other study suggested that intramyocellular lipids were increased after 7 days high-fat diet (38). These studies suggest that a reduction in fat intake reduces intracellular fat accumulation and leads to improved insulin sensitivity.
Dietary fiber can reduce the risk of type 2 diabetes by several mechanisms. Dietary fiber may slow glucose absorption from the intestine, which lowers the glycemic index of carbohydrates (39). Some types of dietary fiber have been pointed out to increase bile acid excretion and increase pool size of bile acids (40). Those increased bile acid increase secretion of glucagon-like peptide 1 via activation of TGR5 and improve glycemic control (41-44). In addition, increased production of short-chain fatty acids by bacterial fermentation of fiber is also thought to have beneficial effects on glucose and energy homeostasis through regulation of intestinal gluconeogenesis (45-47). Previous randomized crossover trials have shown that, compared with a moderate-fiber diet, a high-fiber diet reduced mean daily preprandial plasma glucose concentrations by 13 mg per deciliter (48).
It is also possible that different amino acids have distinct effects on glucose metabolism and insulin resistance (49). In this regard, a randomized crossover trial suggested that partial replacement of meat protein with soy protein improved insulin sensitivity and both total and LDL cholesterol concentrations in humans (50).
The present meta-analysis has several strengths. First, the focus was on clinical trials conducted in patients with type 2 diabetes for at least 4 weeks, which were the minimal time needed to observe meaningful changes in HbA1c levels. Therefore, the beneficial effects of a vegetarian diet on glycemic control seen in this meta-analysis could indicate its utility as a treatment alternative for type 2 diabetes. Second, by focusing on dietary patterns, rather than on isolated nutrients, the major findings are easily applicable to both general and clinical populations (51,52). Third, there was no significant heterogeneity among the controlled trials included in the analysis.
Notwithstanding, the study also has several limitations. First, this meta-analysis used studies with inherent design limitations, particularly small sample sizes. Despite this, changes in HbA1c reached statistical significance. Second, some of the studies were not randomized; even with randomization, baseline between-group differences may have influenced the results, particularly in small trials. Third, the strongest effects on HbA1c were observed in the two nonrandomized studies (17,19). These studies had younger mean ages and higher percentages of women. Perhaps more importantly, the comparison groups in these two studies were not asked to make diet changes and presumably continued their habitual diets. In contrast, three other studies compared vegetarian diets with therapeutic diets specifically designed to improve glycemic control for people with diabetes (15,18,20). Since those comparison diets may have improved diabetes more than typical omnivorous diets would be expected to do, the pooled effect size may have been affected as a result. Fourth, in two trials, less than 20% of the participants had HbA1c levels available. However, the results of removal of one study at a time from the analysis still shows a significant effect. Finally, the designs of the included studies did not permit comparisons of the effects of various types of carbohydrate or of animal versus plant protein. Further studies are needed to explore the relationships between specific foods and glycemic control.
Conclusions
Evidence from clinical trials has shown that vegetarian diets reduce HbA1c levels, suggesting that they may be beneficial in the prevention and management of type 2 diabetes.
Acknowledgements
We thank Richard Holubkov, PhD, of the University of Utah, for consultation regarding statistical analyses.
Financial support for this study was provided by the Japan Society for the Promotion of Science (Grant-in-Aid for JSPS Fellows Grant number 23-10883); and the Nestlé Nutrition Council, Japan.
Authors’ contributions: YY participated in the study design, citation search, and data extraction and analysis, developed figures and tables, and drafted the manuscript; NDB participated in the study design, analysis, and drafting of the manuscript; SML participated in the citation search and data extraction and analysis, and reviewed the manuscript for critical content. MW reviewed the manuscript for critical content.
Disclosure: Drs. Yokoyama and Watanabe report no conflicts of interest. Dr. Barnard writes books and articles and gives lectures related to nutrition and health, and has received royalties and honoraria from these sources. He also serves as president, without financial compensation, of the Physicians Committee for Responsible Medicine, which promotes the use of low-fat, plant-based diets and discourages the use of animal-derived, fatty, and sugary foods. Susan Levin serves as the Director of Nutrition Education for the Physicians Committee for Responsible Medicine.
References
- Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation 1999;100:1132-3. [PubMed]
- American Heart Association. Cardiovascular Disease & Diabetes. Available online: http://www.heart.org/HEARTORG/Conditions/Diabetes/WhyDiabetesMatters/Cardiovascular-Disease-Diabetes_UCM_313865_Article.jsp. Accessed 2014 Aug 4.
- Tonstad S, Stewart K, Oda K, et al. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis 2013;23:292-9. [PubMed]
- Orlich MJ, Fraser GE. Vegetarian diets in the Adventist Health Study 2: a review of initial published findings. Am J Clin Nutr 2014;100:353S-8S. [PubMed]
- Fung TT, Schulze M, Manson JE, et al. Dietary patterns,meat intake,and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164:2235-40. [PubMed]
- Song SJ, Lee JE, Paik HY, et al. Dietary patterns based on carbohydrate nutrition are associated with the risk for diabetes and dyslipidemia. Nutr Res Pract 2012;6:349-56. [PubMed]
- Barnard ND, Katcher HI, Jenkins DJ, et al. Vegetarian and vegan diets in type 2 diabetes management. Nutr Rev 2009;67:255-63. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [PubMed]
- Higgins JP, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available online: www.cochrane-handbook.org
- Follmann D, Elliott P, Suh I, et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769-73. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [PubMed]
- Borenstein M, Hedges L, Higgins J, et al. Comprehensive Meta-analysis Version 2, Biostat, Englewood NJ (2005).
- Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr 2009;89:1588S-1596S. [PubMed]
- de Mello VD, Zelmanovitz T, Perassolo MS, et al. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am J Clin Nutr 2006;83:1032-8. [PubMed]
- Ferdowsian HR, Barnard ND, Hoover VJ, et al. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am J Health Promot 2010;24:384-7. [PubMed]
- Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet Med 2011;28:549-59. [PubMed]
- Mishra S, Xu J, Agarwal U, et al. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study. Eur J Clin Nutr 2013;67:718-24. [PubMed]
- Nicholson AS, Sklar M, Barnard ND, et al. Toward improved management of NIDDM: A randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med 1999;29:87-91. [PubMed]
- Huang W, Castelino RL, Peterson GM. Metformin usage in type 2 diabetes mellitus: are safety guidelines adhered to? Intern Med J 2014;44:266-72. [PubMed]
- Johansen K. Efficacy of metformin in the treatment of NIDDM. Meta-analysis. Diabetes Care 1999;22:33-7. [PubMed]
- Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800-11. [PubMed]
- Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA 1998;280:2001-7. [PubMed]
- Yokoyama Y, Nishimura K, Barnard ND, et al. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med 2014;174:577-87. [PubMed]
- Barnard ND, Scialli AR, Turner-McGrievy G, et al. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am J Med 2005;118:991-7. [PubMed]
- Barnard ND, Scialli AR, Bertron P, et al. Effectiveness of a low-fat vegetarian diet in altering serum lipids in healthy premenopausal women. Am J Cardiol 2000;85:969-972. [PubMed]
- Craig WJ, Mangels AR. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc 2009;109:1266-82. [PubMed]
- Barnard N, Levin S, Trapp C. Meat consumption as a risk factor for type 2 diabetes. Nutrients 2014;6:897-910. [PubMed]
- Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr 2011;94:1088-96. [PubMed]
- Rock CL, Flatt SW, Pakiz B, et al. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care 2014;37:1573-80. [PubMed]
- Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006;29:1777-83. [PubMed]
- Anderson JW, Ward K. High-carbohydrate, high-fiber diets for insulin-treated men with diabetes mellitus. Am J Clin Nutr 1979;32:2312-21. [PubMed]
- Petersen KF, Dufour S, Befroy D, et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664-71. [PubMed]
- Hocking S, Samocha-Bonet D, Milner KL, et al. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev 2013;34:463-500. [PubMed]
- Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005;54:1926-33. [PubMed]
- Goff LM, Bell JD, So PW, et al. Veganism and its relationship with insulin resistance and intramyocellular lipid. Eur J Clin Nutr 2005;59:291-8. [PubMed]
- Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Intramyocellular lipid content and molecular adaptations in response to a 1-week high-fat diet. Obes Res 2005;13:2088-94. [PubMed]
- Livesey G, Tagami H. Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): meta-analysis of randomized controlled trials. Am J Clin Nutr 2009;89:114-25. [PubMed]
- Story JA, Kritchevsky D. Bile acid metabolism and fiber. Am J Clin Nutr 1978;31:S199-S202. [PubMed]
- Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc 2008;108:1716-31. [PubMed]
- Mansour A, Hosseini S, Larijani B, et al. Nutrients related to GLP1 secretory responses. Nutrition 2013;29:813-20. [PubMed]
- Harach T, Pols TW, Nomura M, et al. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep 2012;2:430. [PubMed]
- Watanabe M, Morimoto K, Houten SM, et al. Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS One 2012;7:e38286. [PubMed]
- Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr 2008;138:439-42. [PubMed]
- Jenkins DJ, Kendall CW, Axelsen M, et al. Viscous and nonviscous fibres, nonabsorbable and low glycaemic index carbohydrates, blood lipids and coronary heart disease. Curr Opin Lipidol 2000;11:49-56. [PubMed]
- De Vadder F, Kovatcheva-Datchary P, Goncalves D, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014;156:84-96. [PubMed]
- Chandalia M, Garg A, Lutjohann D, et al. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000;342:1392-8. [PubMed]
- Newsholme P, Cruzat V, Arfuso F, et al. Nutrient regulation of insulin secretion and action. J Endocrinol 2014;221:R105-20. [PubMed]
- van Nielen M, Feskens EJ, Rietman A, et al. Partly Replacing Meat Protein with Soy Protein Alters Insulin Resistance and Blood Lipids in Postmenopausal Women with Abdominal Obesity. J Nutr 2014;144:1423-9. [PubMed]
- Campbell TC. Untold Nutrition. Nutr Cancer 2014;66:1077-82. [PubMed]
- Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3-9. [PubMed]


