Transient post-operative atrial fibrillation predicts short and long term adverse events following CABG
Introduction
Atrial fibrillation (AF) is the most frequent complication following coronary artery bypass graft (CABG) surgery affecting 16-30% of patients in the post-operative period (1,2). Post-operative AF (POAF) is associated with prolonged hospitalization, readmission to the intensive care unit, congestive heart failure, stroke, and an increased utilization of healthcare resources (2-7). The identification of preoperative risk factors, clinical predictive models, and prophylactic strategies to reduce the morbidity and mortality of this common complication remains a priority (8-12).
The incidence of AF in the first year following CABG is more common in patients less than 70 years of age, associated with an increased rate of renal dysfunction and infection (13), and with increased rates of in-hospital and short-term mortality (14). The presence of POAF does confer a protracted risk of morbidity and mortality even if patients are discharged in sinus rhythm. Whether this is related to the substantial rate (39%) of recurrent atrial arrhythmias post operatively (15) is currently unknown.
We sought to determine (I) the long-term consequences associated with transient POAF (TPOAF); and (II) the effect of commonly employed medical therapies on morbidity and mortality in patients with this condition.
Methods
Study design and patient population
The Cleveland Clinic Cardiovascular Information Registry contains prospectively collected clinical and laboratory data of the first thousand patients undergoing open heart surgery each year and maintains complete 30, 60, and 90 days, and 1 year follow-up information. This database was utilized to identify 5,205 consecutive patients without any prior history of documented AF who underwent first time, isolated CABG between January 1993 and December 2005. A total of 1,560 patients were identified with POAF (defined as AF lasting at least 2 minutes but less than 7 days), 70 of which were discharged with persistent AF and excluded from the analysis. Patients diagnosed with the complication of TPOAF (n=1,490, 29%) who were not discharged to home with persistent AF were then compared to those patients that did not develop post-operative AF (n=3,645). Consenting participants were greater than 18 years of age and met no exclusion criteria. Patients were excluded if they were found to have a pre-operative diagnosis of chronic or paroxysmal AF, bleeding diathesis, hypercoagulable state, any requirement for chronic anticoagulation with warfarin, underwent a combined CABG/valve or COX-MAZE procedure, or were identified as having had prior open heart surgery. In addition, patients with a known history of, or preoperative echocardiogram compatible with, significant valvular disease were excluded from the analysis. All operations were performed by experienced cardiothoracic surgeons using standard bypass techniques at their discretion in a large, tertiary referral teaching center. In the immediate post-operative period, all patients were placed on continuous telemetry in the surgical intensive care unit until such time as they were deemed clinically suitable for transfer to a combined cardiology/cardiothoracic surgery step-down unit. Key clinical and laboratory characteristics were prospectively collected in both the pre- and post-operative periods. Post discharge follow-up was obtained through a combination of office visits and telephone interview. Discharge medication prescriptions, including statins, beta blockers, angiotensin converting enzyme inhibitors, aspirin, warfarin, amiodarone, and calcium channel blockers, were also recorded in the database. The study was approved by the institutional review board (IRB) as part of the ongoing CVIR research group.
Endpoints
Patients diagnosed with TPOAF were compared to those patients that did not develop POAF for the individual endpoints of overall death, myocardial infarction (MI), and stroke. The impact of several commonly prescribed classes of discharge medications on these outcomes was also assessed. Data regarding these endpoints were prospectively collected on each patient during their hospitalization and at 30 days, 6 months, and annually up to 5 years thereafter. Median follow-up was 1,880 days from the time of discharge.
Definitions
Patients noted to have any episode(s) of supraventricular tachycardia recorded via bedside telemetry or 12-lead electrocardiogram (EKG) and identified as AF or flutter by a physician were identified as having POAF. TPOAF refers to those patients noted to develop POAF that were not discharged to home with an EKG showing AF. Death is defined as all-cause mortality and was confirmed via social security death index (SSDI) or family telephone interview. Stroke was defined as a new, acute focal neurologic deficit of greater than 24 hours duration thought to be due to ischemia or intracranial hemorrhage and supported by compatible findings on standard imaging modalities such as magnetic resonance imaging, computed tomography, or trans-cranial Doppler. MI was defined as any combination of new “q” waves in 2 contiguous leads on 12-lead EKG, typical rise and fall of cardiac biomarkers (or elevation of biomarkers to 5× upper limit normal in the post-operative setting prior to discharge) in association with compatible clinical presentation, single photon emission tomography, positron emission computed tomography, or trans thoracic echocardiography suggestive of myocardial scarring.
Statistical analysis
Descriptive statistics for continuous and categorical variables were used to summarize baseline clinical and laboratory characteristics-including the discharge medication profile. Differences between the groups were analyzed with the chi-squared or Fischer exact test for categorical variables and the student’s t-test or Wilcoxon rank-sum test for continuous variables. Predictors of long-term mortality were initially individually evaluated and then confirmed with multivariate proportional hazards model. Kaplan-Meier curves were generated to depict survival differences between the groups.
Propensity score (PS) analysis was performed to adjust for baseline characteristics by use of a nonparsimonious logistic regression model. This analysis included relevant variables including: age, sex, race, history of hypertension, diabetes, tobacco use, MI, ventricular tachycardia, peripheral arterial disease, carotid disease, COPD, renal insufficiency, CCS class, NYHA class, and ejection fraction.
Patients were assigned a score from 0-1 based upon probability of developing POAF. The PS was then incorporated into a subsequent proportional hazards model as a covariate and for propensity matching of the cohort. The c-statistic for the logistic regression model was 0.79. Multivariable Cox proportional hazards methods (with stepwise regression) were used to identify variables independently associated with mortality. A multivariate Cox proportional hazards model was utilized to control for baseline inequalities in discharge regimen including use of beta blockers, statins, aspirin, ACE inhibitors, warfarin, anti-arrhythmics, and amiodarone. All analyses were two-tailed and the limit for statistical significance was defined as P value of <0.05. All statistical analysis was performed on SAS version 8.2.
Results
Baseline characteristics
Baseline characteristics comparing patients with TPOAF to those without are presented in Table 1. A total of 1,490 (29%) of the 5,205 patients included in the final analysis were noted to develop TPOAF. The groups were similar with regard to gender and prior history of diabetes, coronary artery disease (CAD), MI, and chronic kidney disease. Patients with TPOAF were older, more often white, hypertensive, and more likely to have a history of PAD, COPD, and carotid disease as compared to patients that did not develop POAF.
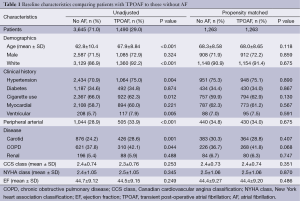
Full table
Discharge medications
Discharge medication usage between the groups is displayed in Table 2. Discharge medication prescription patterns differed between groups as patients with TPOAF were statistically more likely to be treated with warfarin, amiodarone, or other antiarrhythmic therapy.
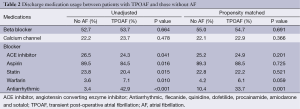
Full table
In-hospital clinical outcomes
Table 3 contains the unadjusted rates of death, MI and stroke. Although there were very few in-hospital deaths (seven total events), patients with TPOAF had a significantly higher unadjusted rate of in-hospital mortality as compared to those without POAF (0.4% vs. 0%; P<0.001). There were no in-hospital strokes or post-operative MIs reported in any of the groups.
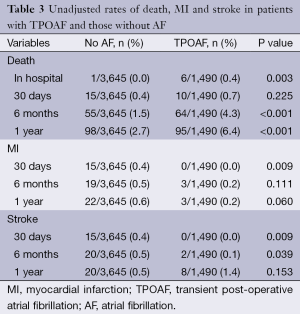
Full table
Clinical outcomes at 1 year
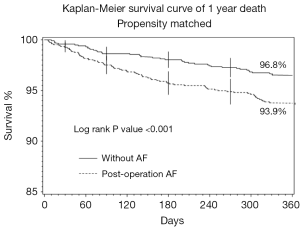
The overall rates of 1-year mortality, MI and stroke were 3.7%, 0.8%, and 2.6% respectively. Patients with TPOAF had a significantly higher incidence of death at 1 year as compared to patients without POAF (6.4% vs. 2.7%; P<0.001). Figure 1 contains the Kaplan-Meier Curve that depicts the overall mortality between the groups at 1 year. There was no significant difference between the groups with respect to rate of MI (0.2% vs. 0.6%; P=0.060) or stroke (0.5% vs. 1.4%; P=0.153) at 1 year of follow-up.
Multivariable analysis
Figure 2 contains the results of the multivariable analysis. After controlling for baseline inequalities, multivariable analysis indicates that TPOAF was an independent predictor of all-cause mortality (HR 1.89, 95% CI, 1.42-2.53; P<0.001) Additional predictors of mortality included age, peripheral vascular disease, renal insufficiency, or prior tobacco abuse. The use of statin and beta blocker therapies were independently associated with a reduction in death at 1 year, however the use of aspirin, warfarin, or amiodarone did not significantly impact overall mortality.
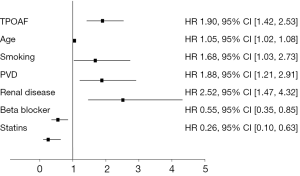
Propensity analysis
Matched cohorts of 1,263 patients with and without TPOAF were subsequently analyzed. After propensity matching there were no significant differences between the groups with regard to age, race, history of hypertension, peripheral vascular disease, ventricular tachycardia or COPD. In the propensity matched cohort, one-year mortality remained significantly higher in the TPOAF group in comparison to those without AF (6.1% vs. 3.2%; P<0.001). Results of the multivariate analysis of the propensity matched cohort suggest that age, tobacco use, and renal disease are predictors of mortality.
After PS analysis was performed, differences in discharge medications still demonstrated that patients in the TPOAF group were more likely to be treated with antiarrhythmic therapy, and there was a non-significant trend toward treatment of these groups with warfarin (Table 2).
Discussion
That POAF following CABG surgery is associated with increased rates of early morbidity has been well established. Patients with POAF are at an increased risk of post-operative hemodynamic instability requiring prolonged mechanical ventilation, increased rates of reintubation, vasopressor medications, and increased length of stay in the costly intensive care unit setting (2,4-6,16). The morbidity of POAF has also been noted to extend beyond the index hospitalization with increased rates of embolic stroke at 30 days, frequent readmission related to recurrent or persistent arrhythmia, and possibly an increase in overall mortality up to 6 months following discharge (1,6,15). Notably, the majority of prior work on this subject has focused upon the in-hospital or short-term outcomes of this population and has been limited by small numbers of patients, incomplete follow-up data, and in many instances involved heterogeneous surgical populations. Therefore, the impact of POAF and its treatment on long-term morbidity and mortality in patients undergoing first time, isolated CABG surgery remains unclear.
The primary results of our study indicate that, in patients undergoing first-time isolated CABG, TPOAF is a common complication in the post-operative period (29%) and is a strong, independent predictor of all-cause, short and long-term mortality. In addition, the use of statin and beta blocker therapies in the post-operative period were independently associated with a reduction in the rate of death of the entire cohort at 1 year, while therapies commonly utilized in the setting of POAF-such as aspirin, warfarin, and amiodarone-did not significantly impact the overall mortality. The results of our study are supported by previous work involving smaller populations followed for shorter periods of time suggesting that POAF is indeed associated with higher rates of in-hospital and short-term mortality (5,13). Our findings also confirm the results of the other studies which document the long-term mortality associated with POAF (14).
The exact mechanism through which TPOAF confers an increased risk of mortality is not readily discernable from our data. As is commonly the case, patients afflicted with TPOAF had a greater number of baseline co-morbidities placing them at higher risk of adverse events. However, given that a significant association between TPOAF and mortality persisted after adjusting for confounding variables, it would appear that the observed excess in mortality are unlikely the result of the differences noted in baseline risk. Alternatively, it is possible that TPOAF is not directly responsible for the observed increase in mortality, but rather, is an epiphenomenon (7,14). By this theory, the relationship between TPOAF and mortality is not causal; rather the development of TPOAF may simply be a marker for a vulnerable sub-population that is subject to a significantly heightened rate of all-cause mortality.
Our finding that the post-operative use of both statins and beta blockers were independently associated with a reduction in 1 year mortality tends to support the theory that key differences in discharge medication profiles may have significantly affected long-term outcome in this population. The mechanism through which beta blockers or statins might convey this benefit remains speculative. Given the accumulating body of data to suggest that AF-especially transient or paroxysmal AF-may be an inflammation-mediated process (8,17-19) and the recent work suggesting that the anti-inflammatory properties of statins may reduce the incidence of TPOAF (12,20-22), it is plausible that the post-operative use of statins could serve to abort or retard recurrent episodes of TPOAF and its attendant morbidity. Likewise, beta blockers have long been utilized in the perioperative setting and are effective in controlling both ventricular and atrial-based arrhythmias, while at the same time reducing myocardial oxygen consumption and ischemic burden (11). Furthermore, several landmark trials have demonstrated a substantial reduction in cardiovascular event rates and an overall improvement in outcomes up to 6 months following surgery associated with the utilization of perioperative beta blockade (23,24). While numerous studies have suggested that preoperative beta blockers are effective in the prevention of POAF, data regarding the post-operative efficacy of these agents is lacking. Given the likelihood of heightened sympathetic tone in post-operative patients with AF, it is possible that this population may stand to gain a substantially greater and more durable benefit from post-operative beta blocker use than was previously realized.
That TPOAF was not associated with an increase in the incidence of stroke-an anticipated consequence of AF-is notable. This finding is in contrast to that of previous work that suggests a significant association between POAF and early or in-hospital stroke-especially in the setting of low cardiac output (14,25,26). However, these studies were almost exclusively limited to the early (<10 days) post-operative period. Our data confirms that of the only other study to evaluate the long-term association between POAF and stroke, which also found no association between POAF and stroke up to 1 year following surgery (13). This finding may be due to the preferential use of both antiarrhythmic and anticoagulant therapy in the POAF groups resulting in higher rates of sinus rhythm maintenance and less cardioembolic stroke. In the current study, patients with TPOAF (29%) were indeed significantly more likely to be discharged on some form of antiarrhythmic therapy as compared to those without POAF. Whether this suggests that TPOAF has a low likelihood of recurrence and therefore a low risk of embolic events or that it is, as previously suggested, simply a marker for a subpopulation vulnerable to noncardiovascular demise is unknown. Furthermore, despite previous reports of a possible survival benefit in patients discharged on antiarrhythmic therapy, our multivariable analysis failed to show a survival advantage with either antiarrhythmic or warfarin therapy (14).
Study limitations
There are several limitations of the present study. Firstly, this data is subject to all the limitations inherent to any single-center, retrospective, observational study. Secondly, we are unable to verify that patients were compliant with, or did not have changes made to, the various classes of agents contained in their discharge medication regimen. As a result, it is possible that our data may over or underestimate any possible therapeutic effect. Furthermore, given the long-term nature of follow-up and the large referral population contained in this database, it was not technically feasible to objectively assess patients for the recurrence of AF. In addition, due to the telephone method of follow-up and the paucity of autopsy data, we are unable to comment on the specific cause of death that was associated with TPOAF. We did observe that vast majority of patients with POAF had only transient episodes-with only 70 patients (1.3%) being discharged in persistent AF. We view this as a strength of the study given that the vast majority of patients encountered in the post-operative setting appear to have TPOAF and no prior work has reported on this specific subgroup in regards to outcome or treatment strategy.
Conclusions
The present study suggests that TPOAF complicating first-time isolated CABG surgery may be an independent predictor of all-cause, long-term mortality. In addition, our study suggests that the use of both statin and beta-blocker therapies can significantly reduce this risk, while commonly used anti-arrhythmic and anticoagulant medications do not significantly impact overall mortality. These results provide some evidence of the benefit of statins and beta blockers beyond the perioperative setting and suggest that the development of TPOAF identifies a population at elevated risk for death not only in the immediate or short term, but for a prolonged period following the index hospitalization. These data warrant prospective validation through appropriately powered randomized trials.
Acknowledgements
The authors gratefully acknowledge Drs Peter Zimbwa, Stephen Chen, and Ryan Daly for their assistance with data collection.
Disclosure: The authors declare no conflict of interest.
References
- Lauer MS, Eagle KA, Buckley MJ, et al. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis 1989;31:367-78. [PubMed]
- Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter Study of Perioperative Ischemia Research Group. JAMA 1996;276:300-6. [PubMed]
- Ferguson TB Jr, Hammill BG, Peterson ED, et al. A decade of change--risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990-1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg 2002;73:480-9; discussion 489-90. [PubMed]
- Creswell LL, Schuessler RB, Rosenbloom M, et al. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 1993;56:539-49. [PubMed]
- Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg 1997;226:501-11; discussion 511-3. [PubMed]
- Tamis JE, Steinberg JS. Atrial fibrillation independently prolongs hospital stay after coronary artery bypass surgery. Clin Cardiol 2000;23:155-9. [PubMed]
- Hogue CW Jr, Creswell LL, Gutterman DD, et al. Epidemiology, mechanisms, and risks: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest 2005;128:9S-16S. [PubMed]
- Halonen J, Halonen P, Järvinen O, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA 2007;297:1562-7. [PubMed]
- White CM, Caron MF, Kalus JS, et al. Intravenous plus oral amiodarone, atrial septal pacing, or both strategies to prevent post-cardiothoracic surgery atrial fibrillation: the Atrial Fibrillation Suppression Trial II (AFIST II). Circulation 2003;108 Suppl 1:II200-6. [PubMed]
- Fortescue EB, Kahn K, Bates DW. Development and validation of a clinical prediction rule for major adverse outcomes in coronary bypass grafting. Am J Cardiol 2001;88:1251-8. [PubMed]
- Halonen J, Hakala T, Auvinen T, et al. Intravenous administration of metoprolol is more effective than oral administration in the prevention of atrial fibrillation after cardiac surgery. Circulation 2006;114:I1-4. [PubMed]
- Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation 2006;114:1455-61. [PubMed]
- Elahi M, Hadjinikolaou L, Galiñanes M. Incidence and clinical consequences of atrial fibrillation within 1 year of first-time isolated coronary bypass surgery. Circulation 2003;108 Suppl 1:II207-12. [PubMed]
- Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol 2004;43:742-8. [PubMed]
- Loubani M, Hickey MS, Spyt TJ, et al. Residual atrial fibrillation and clinical consequences following postoperative supraventricular arrhythmias. Int J Cardiol 2000;74:125-32. [PubMed]
- Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 1996;94:390-7. [PubMed]
- Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J 2006;27:136-49. [PubMed]
- Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J 2005;26:2083-92. [PubMed]
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104:2886-91. [PubMed]
- Ramani G, Zahid M, Good CB, et al. Comparison of frequency of new-onset atrial fibrillation or flutter in patients on statins versus not on statins presenting with suspected acute coronary syndrome. Am J Cardiol 2007;100:404-5. [PubMed]
- Tveit A, Grundtvig M, Gundersen T, et al. Analysis of pravastatin to prevent recurrence of atrial fibrillation after electrical cardioversion. Am J Cardiol 2004;93:780-2. [PubMed]
- Shiroshita-Takeshita A, Schram G, Lavoie J, et al. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation 2004;110:2313-9. [PubMed]
- Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996;335:1713-20. [PubMed]
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999;341:1789-94. [PubMed]
- Hogue CW Jr, Murphy SF, Schechtman KB, et al. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999;100:642-7. [PubMed]
- Stamou SC, Hill PC, Dangas G, et al. Stroke after coronary artery bypass: incidence, predictors, and clinical outcome. Stroke 2001;32:1508-13. [PubMed]


