Magnetic resonance imaging as a tool to assess reliability in simulating hemodynamics in cerebral aneurysms with a dedicated computational fluid dynamics prototype: preliminary results
Introduction
The concept of using medical image data for the creation of computational models and the subsequent simulation of hemodynamics in these models has been expressed already almost 20 years ago (1,2). Around a decade ago, this concept was revived mainly because of the progress in computational hardware which enables the researcher to now conduct these simulations on personal computer workstation with cost-effective, versatile software that is flexible enough to be used with computational models derived from medical image data for a specific patient (3). Since then, a variety of studies have been published exploring the feasibility of using computational fluid dynamics (CFD) for the simulation and quantification of aneurysm hemodynamics as well as investigating its usefulness for therapeutic applications.
In cerebral aneurysms, geometrical properties of the parent artery (4-6) were reported to influence the simulation results as did variations in the inflow waveform of the boundary conditions (7,8), and the duration of the simulated cardiac cycle (9). Hemodynamic conditions in the parent artery were potentially associated with aneurysm formation (10) as were hemodynamics values in the aneurysm itself with risk of rupture (11-14). Validation of simulation results was attempted using fluoroscopic angiographic images (15,16) or phase contrast magnetic resonance imaging (pcMRI). For the latter, first 2D methods were explored (17,18) and, later, the time-dependent velocity fields obtained with the novel 4D pcMRI methods were correlated with corresponding quantities obtained with the CFD simulations (19,20). In addition, CFD methods have been employed to investigate the potential of this technique for predicting hemodynamics changes and to explain different outcome after endovascular treatment with the novel flow diverter devices (21-23).
All these efforts contributed to translating CFD as an engineering technology into a clinical research tool. While the high potential of CFD for the quantification and visualization of cerebral aneurysm hemodynamics has been established in the last decade, recent discussions show that a better understanding and a thorough validation of this technique is needed, before its potential value for therapeutic decisions can be assessed (24-26). In addition to validating software performance and reproducibility of simulation results, it is important to understand limitations for the application of these simulation techniques to in vivo data. In this study we compare measurements of velocity patterns using a 2D phase contrast magnetic resonance method with results of a novel CFD prototype system.
Materials and methods
2D pcMRI
The 2D pcMRI images were acquired at a 1.5 T MRI scanner (Siemens AG, Erlangen) from six patients diagnosed with a cerebral aneurysm. From a 3D time-of-flight (TOF) scan, which was acquired as a localizer (FOV 220 mm, matrix: 512×256, slice thickness: 0.5 mm), cross-sectional planes intersecting each aneurysm at approximately two perpendicular orientations were selected. Dependent on the length of the cardiac cycle, 12-20 pcMRI images per cardiac cycle were obtained using a peripheral monitor and a retrospective gating approach [FOV 160 mm, slice thickness 5 mm, matrix: 256×192, velocity-encoding (VENC) values, perpendicular to scan plane only, ranged from 60-150 cm/s]. Images at systole were selected for comparison with CFD results.
CFD simulations
CFD, as a branch of fluid dynamics, utilizes numerical methods to solve problems which involve fluid flows. Computational algorithms which approximate the real system and which use boundary conditions that define the geometry, the inflow and outflow parameters of the model, calculate the velocity vector field and other derived hemodynamic parameters such as pressures and wall shear stresses, i.e., forces, which the fluid exerts onto the wall. As a first step in this process, the physical bounds of the computational model are defined. This so defined volume is then divided into small elements (cells) that constitute the computational mesh. The governing physical equations, for this case, the Navier-Stokes equations, are then iteratively solved on the computational mesh taking into consideration the boundary conditions. Post-processing software is then utilized for further analysis and visualization.
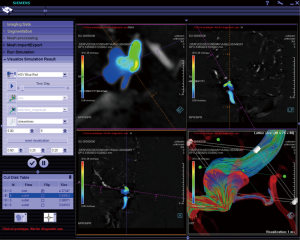
IRB approval was obtained for this study. The 3D digital angiography subtraction (DSA) image data, originally acquired during a diagnostic angiogram, were retrospectively collected for the six aneurysms. Image data was transferred to the dedicated workstation of a CFD research prototype (Siemens AG, Figure 1). While client-server systems have been recently described as a model for integration into the clinical workflow (27), the CFD research prototype described here consisted of stand-alone software installed on a single workstation with computational power similar to a personal computer. The software as well as the entire prototype is currently developed as a research tool and not part of any commercial clinical software package. An optimized algorithm consisting of different parts was utilized to conduct the CFD simulations using this prototype: in part 1, a 3D surface model of the corresponding vasculature was created. In part 2, small arterial branches and venous contamination (if present) were manually eliminated. In part 3, the computational mesh was created after manually defining inflow and outflow regions. In part 4, steady CFD simulations were performed (maximum inflow velocity 0.8 m/s). Using a level-set based embedded boundary method, the Navier-Stokes equations were solved with user-specified boundary conditions (28). Blood was modeled as an incompressible fluid with a density of 1,000 kg/m3 and a viscosity of 0.004 kg/ms. In part 5, simulation results were stored on disk using the visualization toolkit (vtk) file format (Kitware Inc.) and cross sections were defined across the computational model at the same locations as the cross sections measured with 2D pcMRI. At these cross sections, velocity components perpendicular to each cross section were visualized in a similar fashion as the measured velocity components.
Results
In all six cases, generally a good agreement was found between the major flow features in the cross sections calculated with the CFD prototype and measured with 2D pcMRI. A more detailed discussion for each case follows (please also refer to Figure 2).
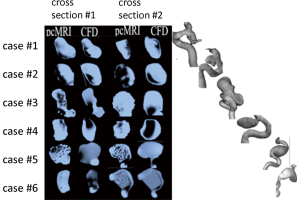
Case 1
In the first cross section, the bilobular or dumbbell shape of the aneurysm shows regions of opposite flow directions at the base of the dumbbell relative to both lobes for both simulated and measured data. Qualitatively higher velocities were visible in the simulated case compared to the measurements. The velocity overestimation in the simulation might be due to the assumption of rigid walls, which may be less valid for the lobes, as these were found to exhibit higher wall pulsatility then the main body of the aneurysm (29). In the second cross section, opposite flow directions are visible at the left and right wall indicating a circular blood motion typically found in cerebral aneurysms. Excellent qualitative agreement between the major flow features in this cross section was apparent.
Case 2
Both the simulated as well as the measured data agreed in the overall direction of blood flow, however, the shape and extend of the major flow features differ considerably in both cross sections. Particularly in the second cross section, the area represented by low voxel intensity was underestimated in the simulations.
Case 3
In this large and irregularly shaped aneurysm, major flow features were well represented by the simulation results in comparison to the 2D pcMRI measurements. Low signal to noise in the 2D pcMRI images at regions of slow flow (potentially more pronounced in this large aneurysms compared to the other ones of smaller diameter) were appreciated.
Case 4
Agreement was overall found favorable in the velocity patterns visible in both cross sections between measurement and simulations sections despite some apparent differences towards the aneurysm dome, in particular in the first cross section. Opposite regions of flow were quite distinguishable in the 2D pcMRI inside the entire dome while in the simulation results, a more diffuse, slow flow pattern was attributed to the dome region. In the second cross section, perpendicular to the first, agreement was better concerning the shapes of the velocity patterns while the simulation seemed to underestimate blood flow velocities.
Case 5
In this large aneurysm, focal areas of high velocity blood flow were well reproduced in the simulation results compared to the measurements. Low signal to noise corresponding to regions of slow velocities were again appreciated similar to case 3.
Case 6
In this aneurysm, which exhibited a thrombosed dome region, velocity patterns agreed very well in the second cross section while also qualitatively moderate to good agreement was found in the first cross section where the CFD simulation overestimated velocity magnitudes.
Discussion
Illustrated by the qualitative comparison of in vivo measurements realized by 2D pcMRI and steady-inflow CFD simulations performed with a dedicated CFD prototype, CFD as implemented as a dedicated prototype system is capable of reproducing major flow features in cerebral aneurysms.
The findings of this preliminary results study is in good agreement with previously published work using the commercially available CFD solver software (30). In contrast to a commercial software, which, due to its high flexibility in the kinds of simulations it can perform, most often is of high complexity perhaps unsuitable for easy use in a clinical research setting, the application of the CFD prototype system employed here, was very easy to use for the six described cases. The workflow was predefined and segmentation as well as definition of boundary conditions for the simulations was greatly simplified. As costs of this simplification, currently only zero pressure boundary conditions are available and inflow can only be defined by a velocity value. Thus, no pressure values or a velocity profile defined across the diameter of the parent artery are currently feasible.
In this preliminary results study, steady simulations were chosen as only these are from a practical clinical standpoint sufficiently fast to provide results within the time frame of a diagnostic angiogram or during an endovascular embolization procedure. Despite this simplified approach, the qualitative agreement between simulation and measurement was generally very good in that it provided quickly information for the intra-aneurysmal blood flow patterns. It may be argued that this information may be too cursory and more sophisticated simulations are needed utilizing time-varying inflow (and even perhaps) outflow conditions. To address this issue, it would be also necessary to define expectations for the CFD simulation results: while the possibility of calculating an aneurysm rupture risk has been discussed as a potential application for CFD, it should be kept in mind that in addition to geometrical and morphological factors giving rise to mechanical forces (31,32), also other factors, such as current smoking, hypertension, family history of stroke other than subarachnoid hemorrhage, hypercholesterolemia and regular physical exercise have been implicated to attribute to aneurysm rupture risk (33). While hypertension might be related to elevated wall shear stress, a hemodynamic parameter accessible via CFD, it might be difficult to account for the remaining factors in the computational simulations.
Other clinical research applications of CFD have recently been reported which investigate entirely mechanical effects, e.g., the influence of eight different configurations of stenting for bifurcation aneurysms on the hemodynamics inside the aneurysm (34). The authors demonstrated that the crossing-Y and kissing stent configuration lead to lower flow velocity within the aneurysms. In another study, CFD simulations helped to predict the rupture side in subarachnoid hemorrhage in a patient with bilateral vertebral aneurysms in order to decide which aneurysm need to be treated first (35). These are examples of scenarios where CFD was used to address a well-defined clinical research issue, and these kinds of scenarios may be readily addressed by the CFD research prototype describe here.
Conclusions
CFD simulations of cerebral aneurysms is an evolving technology with promising applications in clinical research for the near future. A standardized approach, as realized by the dedicated CFD prototype system described in this study, will be beneficial for comparison and exchange of simulation results.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Foutrakis GN, Burgreen G, Yonas H, et al. Construction of 3-D arterial volume meshes from magnetic resonance angiography. Neurol Res 1996;18:354-60. [PubMed]
- Ortega HV. Computer simulation helps predict cerebral aneurysms. J Med Eng Technol 1998;22:179-81. [PubMed]
- Steinman DA, Milner JS, Norley CJ, et al. Image-based computational simulation of flow dynamics in a giant intracranial aneurysm. AJNR Am J Neuroradiol 2003;24:559-66. [PubMed]
- Hoi Y, Meng H, Woodward SH, et al. Effects of arterial geometry on aneurysm growth: three-dimensional computational fluid dynamics study. J Neurosurg 2004;101:676-81. [PubMed]
- Castro MA, Putman CM, Cebral JR. Computational fluid dynamics modeling of intracranial aneurysms: effects of parent artery segmentation on intra-aneurysmal hemodynamics. AJNR Am J Neuroradiol 2006;27:1703-9. [PubMed]
- Meng H, Wang Z, Kim M, et al. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: implications of intravascular stenting. AJNR Am J Neuroradiol 2006;27:1861-5. [PubMed]
- Karmonik C, Yen C, Grossman RG, et al. Intra-aneurysmal flow patterns and wall shear stresses calculated with computational flow dynamics in an anterior communicating artery aneurysm depend on knowledge of patient-specific inflow rates. Acta Neurochir (Wien) 2009;151:479-85; discussion 485. [PubMed]
- Karmonik C, Yen C, Diaz O, et al. Temporal variations of wall shear stress parameters in intracranial aneurysms--importance of patient-specific inflow waveforms for CFD calculations. Acta Neurochir (Wien) 2010;152:1391-8; discussion 1398. [PubMed]
- Jiang J, Strother C. Computational fluid dynamics simulations of intracranial aneurysms at varying heart rates: a “patient-specific” study. J Biomech Eng 2009;131:091001. [PubMed]
- Mantha A, Karmonik C, Benndorf G, et al. Hemodynamics in a cerebral artery before and after the formation of an aneurysm. AJNR Am J Neuroradiol 2006;27:1113-8. [PubMed]
- Cebral JR, Castro MA, Burgess JE, et al. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am J Neuroradiol 2005;26:2550-9. [PubMed]
- Jou LD, Lee DH, Morsi H, et al. Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. AJNR Am J Neuroradiol 2008;29:1761-7. [PubMed]
- Tremmel M, Dhar S, Levy EI, et al. Influence of intracranial aneurysm-to-parent vessel size ratio on hemodynamics and implication for rupture: results from a virtual experimental study. Neurosurgery 2009;64:622-30; discussion 630-1. [PubMed]
- Sforza DM, Putman CM, Scrivano E, et al. Blood-flow characteristics in a terminal basilar tip aneurysm prior to its fatal rupture. AJNR Am J Neuroradiol 2010;31:1127-31. [PubMed]
- Ford MD, Stuhne GR, Nikolov HN, et al. Virtual angiography for visualization and validation of computational models of aneurysm hemodynamics. IEEE Trans Med Imaging 2005;24:1586-92. [PubMed]
- Sun Q, Groth A, Aach T. Comprehensive validation of computational fluid dynamics simulations of in-vivo blood flow in patient-specific cerebral aneurysms. Med Phys 2012;39:742-54. [PubMed]
- Karmonik C, Klucznik R, Benndorf G. Blood flow in cerebral aneurysms: comparison of phase contrast magnetic resonance and computational fluid dynamics--preliminary experience. Rofo 2008;180:209-15. [PubMed]
- Boussel L, Rayz V, Martin A, et al. Phase-contrast magnetic resonance imaging measurements in intracranial aneurysms in vivo of flow patterns, velocity fields, and wall shear stress: comparison with computational fluid dynamics. Magn Reson Med 2009;61:409-17. [PubMed]
- Jiang J, Johnson K, Valen-Sendstad K, et al. Flow characteristics in a canine aneurysm model: a comparison of 4D accelerated phase-contrast MR measurements and computational fluid dynamics simulations. Med Phys 2011;38:6300-12. [PubMed]
- Berg P, Stucht D, Janiga G, et al. Cerebral Blood Flow in a Healthy Circle of Willis and Two Intracranial Aneurysms: Computational Fluid Dynamics Versus 4D Phase-Contrast Magnetic Resonance Imaging. J Biomech Eng 2013. [Epub ahead of print]. [PubMed]
- Chong W, Zhang Y, Qian Y, et al. Computational hemodynamics analysis of intracranial aneurysms treated with flow diverters: correlation with clinical outcomes. AJNR Am J Neuroradiol 2014;35:136-42. [PubMed]
- Kulcsár Z, Augsburger L, Reymond P, et al. Flow diversion treatment: intra-aneurismal blood flow velocity and WSS reduction are parameters to predict aneurysm thrombosis. Acta Neurochir (Wien) 2012;154:1827-34. [PubMed]
- Cebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol 2011;32:27-33. [PubMed]
- Kallmes DF. Point: CFD--computational fluid dynamics or confounding factor dissemination. AJNR Am J Neuroradiol 2012;33:395-6. [PubMed]
- Cebral JR, Meng H. Counterpoint: realizing the clinical utility of computational fluid dynamics--closing the gap. AJNR Am J Neuroradiol 2012;33:396-8. [PubMed]
- Strother CM, Jiang J. Intracranial aneurysms, cancer, x-rays, and computational fluid dynamics. AJNR Am J Neuroradiol 2012;33:991-2. [PubMed]
- Schoenhagen P, Zimmermann M, Falkner J. Advanced 3-D analysis, client-server systems, and cloud computing-Integration of cardiovascular imaging data into clinical workflows of transcatheter aortic valve replacement. Cardiovasc Diagn Ther 2013;3:80-92. [PubMed]
- Mihalef V, Ionasec RI, Sharma P, et al. Patient-specific modelling of whole heart anatomy, dynamics and haemodynamics from four-dimensional cardiac CT images. Interface Focus 2011;1:286-96. [PubMed]
- Karmonik C, Diaz O, Grossman R, et al. In-vivo quantification of wall motion in cerebral aneurysms from 2D cine phase contrast magnetic resonance images. Rofo 2010;182:140-50. [PubMed]
- Karmonik C, Klucznik R, Benndorf G. Blood flow in cerebral aneurysms: comparison of phase contrast magnetic resonance and computational fluid dynamics--preliminary experience. Rofo 2008;180:209-15. [PubMed]
- Yasuda R, Strother CM, Taki W, et al. Aneurysm volume-to-ostium area ratio: a parameter useful for discriminating the rupture status of intracranial aneurysms. Neurosurgery 2011;68:310-7; discussion 317-8. [PubMed]
- Mehan WA Jr, Romero JM, Hirsch JA, et al. Unruptured intracranial aneurysms conservatively followed with serial CT angiography: could morphology and growth predict rupture? J Neurointerv Surg 2013. [Epub ahead of print].
- Vlak MH, Rinkel GJ, Greebe P, et al. Independent risk factors for intracranial aneurysms and their joint effect: a case-control study. Stroke 2013;44:984-7. [PubMed]
- Kono K, Terada T. Hemodynamics of 8 different configurations of stenting for bifurcation aneurysms. AJNR Am J Neuroradiol 2013;34:1980-6. [PubMed]
- Kono K, Shintani A, Fujimoto T, et al. Stent-assisted coil embolization and computational fluid dynamics simulations of bilateral vertebral artery dissecting aneurysms presenting with subarachnoid hemorrhage: case report. Neurosurgery 2012;71:E1192-200; discussion E1200-1.


