Fast in vivo quantification of T1 and T2 MRI relaxation times in the myocardium based on inversion recovery SSFP with in vitro validation post Gd-based contrast administration
Introduction
Balanced steady state free precession (bSSFP) sequences (also known by vendor-specific acronyms such as b-FFE, FIESTA or TrueFISP) have found widespread use in applications where rapid imaging is needed, in particular for cardiac imaging (1-3). An advantage of these sequences is their high signal-to-noise (SNR) efficiency. In contrast to conventional turbo spin-echo (TSE) sequences, the steady-state image contrast is not predominantly dominated by a single parameter such as proton density, T1 or T2 relaxation times; rather it is a function of proton density and the T2/T1 ratio. As a consequence, high signal is usually obtained from fluid compartments due to their relatively high T2 values (4). Steady state is reached after a certain transition period. Earlier implementations of this sequence achieved a smooth signal time course towards steady state by preparation of a radio frequency pulse (RF) of flip angle-α/2 at a time interval of half the repetition time (TR) prior to the first α pulse (5). This preparation only works for on-resonance spins. Current implementations use a series of preparation pulses for optimizing the transition to steady state (6).
For magnetization prepared by an inversion pulse prior to a continuous bSSFP readout, Schmitt et al. have derived analytical expressions for the direct calculation of T1, T2 and proton density from a single inversion recovery (IR) bSSFP time-intensity curve (7). Validation was provided with phantom measurements and in vivo imaging: T1, T2 and proton-density-weighted images of the brain derived from T1, T2 and proton density maps obtained with this method compared favorably to conventional T1, T2 and proton density weighted images obtained with clinical TSE techniques (8). Here we present in vitro validation and in vivo application of a technique based on segmented IR balanced steady state free precession (IR-bSSFP) to simultaneously measure T1 and T2 relaxation times of the myocardium in vivo within clinically acceptable time frames.
Potential clinical applications of this technique consist in quantifying myocardial disease where T1 or T2 are subject to change with disease progression. For T1 changes post Gd-based contrast injection, of particular interest is the presence of fibrosis in the heart, as a number of different cardiac pathologies seem to be caused by a common fibrotic process (9). Recent studies indicate that dilated, ischaemic and hypertrophic cardiomyopathies (a genetic disorder with a high incidence of sudden cardiac death in preadolescent and adolescent children) are all associated with raised levels of TGF-β1, a growth factor that is thought to be partially mediating fibrosis (9,10). Changes in T2 are thought to occur with the administration of vasodilatory drugs, such as dipyridamole and adenosine, which increase coronary blood flow in the healthy myocardium and produces changes in tissue blood volume and blood oxygenation. Another potential application for myocardial T2 measurements is the assessment of ischemic disease and the quantification of fat tissue within the heart.
Materials and methods
Imaging setup
In total, 24 agarose solutions (1%) were created containing varying concentrations of Omniscan (0.25-2 mg/mL) and copper-sulfate (0.52-22.17 mg/mL). Solutions were allowed to solidify in 8 mm (inner diameter) test tubes and mounted on a styrofoam holder.
Approval of the institutional review board was obtained for the in vivo imaging portion of this study. Subjects were five healthy volunteers (three male, two female, mean age 55±32 years) with no known history of cardiovascular disease. Images (single slice) were acquired during the clinical examination and retrospectively selected for inclusion in this study. As a consequence, the wait time of the image acquisition after Gd-based contrast injection varied in between subjects.
In vitro imaging (single slice) was performed with the standard 12-channel head matrix coil, and in vivo imaging was performed with a standard body matrix coil. All imaging was performed on a 1.5 T whole-body clinical scanner (MAGNETOM Avanto, Siemens AG, Healthcare Sector, Erlangen, Germany) in separate imaging sessions.
TSE sequence parameters for T1 and T2 measurements
T1 and T2 values for the in vitro samples were determined from images obtained in two separate series using TSE. While the standard single-echo Carr-Purcell-Meiboom-Gill (CPMG) method should be considered the gold-standard for MRI relaxometry, previous studies have demonstrated that relaxometry results obtained with TSE for T2 compare well with CPMG (11) while reducing acquisition time by a factor of 5. In series 1, seven datasets [axial orientation, five slices covering the agarose gel in the test tubes, matrix 128×256, field of view (FOV) 110 mm × 220 mm, in-plane resolution 0.86 mm × 0.86 mm, slice thickness 5 mm, echo time (TE) 10 ms, turbo factor 16] were acquired with the following TR values: 350, 700, 1000, 2000, 3000, 4000 and 5000 ms. The total acquisition time was 25 min.
In series 2, a series of sixteen datasets (spatial parameters and turbo factor as for series 1, TR 3,000 ms) was acquired with the following TE values: 10, 20, 30, 40, 49, 59, 69, 79, 89, 99, 109, 119, 129, 138, 148 and 158 ms; total acquisition time 11 min.
The T1 and T2 values for each sample were determined from the average time-intensity curve of all voxels in a region of interest (ROI) placed in the center of the test tube containing the agarose sample. An in-house developed plugin for ImageJ (NIH, version 1.43k) together with the ImageJ ‘Curve Fitting’ class was used to fit the conventional TSE relaxation equations (12) to the ROI time-intensity curves. For the determination of T2, if ROI signal intensity was less than 10% of the maximum image intensity or if it was less than the image noise (determined as standard deviation from the intensities of all voxels for a ROI placed outside the test tubes in air), this intensity value was not included in the fit (assume transverse magnetization had decayed completely). For the determination of T1, image noise was subtracted from ROI time-intensity curves prior to fitting to account for the Rician nature of noise in the magnitude MRI images (13). For practical considerations, this subtraction yielded only a small correction (less than 10%).
IR-bSSFP sequence parameters for T1 and T2 measurements
For in vivo images, the acquisition matrix was 136×256, number of segments 20, with eight heart beats/slice and a temporal distance of 53 ms between adjacent frames. In Table 1, total scan time, the nominal acquisition window, the cardiac number of images, the average duration of the cardiac cycle are given for each subject. In the time period between the nominal acquisition window and twice the RR interval, no image data was acquired. Recovery curves were sampled for two cardiac intervals. Consecutive acquisitions were started with every second trigger. The flip angle was 50 degrees and images were acquired in a 4-chamber long-axis image orientation with 6 mm slice thickness and a FOV of 425 mm. Parameters for the acquisition of the in vitro IR-bSSFP images as follows: acquisition matrix 384×512, 5 or 10 segments, resulting in a temporal distance between adjacent frames of 13 or 35 ms, flip angle 50 degrees, duration of simulated cardiac cycle: 1,000 ms, trigger every 2nd cycle, 7 mm slice thickness, FOV 210 mm, TE =1.1 ms, pixel bandwidth: 975 Hz. Relaxation delay for the in vitro acquisition was twice the simulated cardiac cycle, i.e., 2,000 ms to closely replicate the situation for the in vivo measurements. For in vitro samples, ROI time-intensity curves were determined as described for TSE acquisitions. Due to the heart motion, the ROI was adjusted for each time frame. For in vivo images, ROIs were defined as (I) the entire wall of left ventricle (LV), (II) an area contained within the interventricular septum (IVS) and (III) an area contained within the LV lateral wall. To minimize the influence of partial volume effects, voxels at the border of the myocardium were not included.
Full table
To extract T1 and T2 values, the following single exponential recovery approximation (7) was fitted to the IR-bSSFP time-intensity curves using an ImageJ plugin developed in-house:
[1] |
From the fit results, both T1 and T2 were calculated according to:
[2] |
[3] |
[4] |
Here, α is the flip angle and INV and T1* are fitting parameters in the first two equations used to calculate T1 and T2 as indicated in the remaining two equations. T1 and T2 values determined in this way were compared to T1 and T2 values from TSE by means of the Pearson correlation coefficient and Bland-Altman plots.
Spin system simulation of longitudinal magnetization recovery
The evolution of the magnetization during a bSSFP readout can be calculated using the following recursive equation (neglecting off-resonance effects) (14):
| Mn=Rx(±∝)[E2(TR, T1, T2)Mn-1+E1(TR, T1)] | [5] |
where Mn is the magnetization vector directly after the nth pulse and Rx(±α) is a rotation matrix about the X-axis in the rotating frame corresponding to an RF excitation with flip angle α (an inversion pulse is represented by α=π). E1 and E2 are the matrix representations of T1 and T2 relaxation:
[6] |
[7] |
The above equations were implemented in Matlab (Mathworks, Inc) and spin system simulations were performed for sample #4, with T1=334 ms and T2=50 ms (values obtained from the TSE measurements). The temporal distance between IR pulses was chosen similar to the in vitro MRI protocol (2,000 ms).
This sample was chosen as its T1 and T2 values were close to reported in vivo values for the myocardium with about 10 min delay after injection of a Gadolinium-based contrast agent (15,16). The steady state magnetization (M0) for this simulation was estimated from the grayscale intensity of the acquired bSSFP images at long T1 times. As a quantitative measure for the agreement between simulations and measurement, absolute relative mean differences (difference at each data point divided by the average of simulation value and measurement value) were calculated.
Repeatability for the in vivo measurements
To establish a measure for the robustness of the here presented methodology for in vivo application, ROI outlines were repeated for a total of five measurement at each anatomical location for subject #3.
Results
In vitro TSE and IR-bSSFP comparison
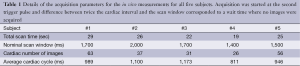
From TSE, T1 values ranged from 78 to 2,760 ms; T2 values from 9 to 91 ms. For T1, good agreement between the TSE and IR-bSSFP results was observed (R=0.88, Figure 1) for T1 values smaller than 1,250 ms. For longer T1 values, IR-bSSFP systematically underestimated T1 with higher deviations for larger values (Figure 1B,D). For T2, excellent agreement between TSE and IR-bSSFP was found for the entire range (R=0.97, Figure 1C,E).
Spin system simulations, single-exponential approximation and in vitro measurement
Very good agreement between the measured IR-bSSFP time-intensity curve for sample #4 (T1=334 ms and T2=50 ms), the single-exponential recovery approximation from Eq. [1] and the spin system simulations were found (Figure 1F). Largest deviations were apparent in the regions where the longitudinal magnetization exhibits a sign change (negative to positive transition, Figure 1F). Absolute mean difference between measurements and spin system simulation was 14.4% and between measurements and Eq. [1] was 10.7%.
In vivo measurement of T1 and T2
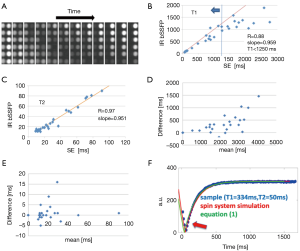
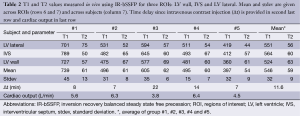
Delay after contrast injection (Δt) for acquisition of IR-bSSFP images ranged from 7 to 22 min (average: 11.6 min, Table 1). In all subjects, IR-bSSFP images exhibited pronounced contrast changes during the IR signal evolution. In early images, contrast between LV myocardium and blood was poor, followed by images in which signal from blood was nulled (due to its shorter T1 value relative to LV myocardium). In later images, contrast between LV myocardium (dark) and blood (bright) was reversed (Figure 2A). Good fits between Eq. [1] and time-intensity curves were obtained for all ROIs (Figure 2B). Average T1 value over all subjects and ROIs was 546±32 ms and average T2 was 59±9 ms. Inter-subject variations in T1 were higher (subject #1 average T1 was 739 ms compared to subject #5 average T1 of 397 ms) than variations across ROIs for each subject. Inter-subject variations in T2 were comparable to variations across ROIs for each subject (Table 2). The pharmacokinetics of the contrast agent and therefore Δt are known to affect T1 (wash-in and wash-out effects). The average T1 values for four out of the five subjects followed a linear relationship with Δt (R=0.87, Figure 2C). The average T1 values for subject #1 deviated strongly from this relationship. No relationship between average T1 and cardiac output (Table 2) was found.

Full table
Repeatability for the in vivo measurements
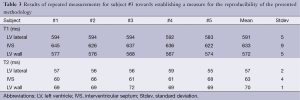
Results for the repeated ROI outlines in subject #3 (in total five measurements at each anatomical location) demonstrate a relatively good reproducibility. Standard deviations for T1 did not exceed 9 ms, and for T2 did not exceed 4 ms. The high contrast of the myocardium relative to the surrounding parenchyma resulted in unambiguous ROI definitions. Highest variation was found for the IVS (Table 3).
Full table
Discussion
In their study of direct T2 quantification of myocardial edema in acute ischemic injury, Verhaert et al. reported an average T2 value for healthy volunteers (n=21) of 55.5±2.3 ms obtained with a T2-weighted breath-hold (12-15 sec) TSE short tau IR sequence (17). This average T2 value as well as the magnitude of its standard deviation compares favorably with the value reported here (59±3 ms) but is should be emphasized that values by Verhaert et al. were obtained without contrast administration.
In their comparison of gadobenate (Gd-BOPTA, 0.1 mmol/kg body weight) with gadopentetate dimeglumine (Gd-DTPA, 0.2 mmol/kg body weight) for assessing myocardial viability in 26 patients, Krombach et al. reported T1 values for remote myocardium (i.e., not infarcted myocardium) of 358±78 and 562±108 ms for 3 min after and 25 min after contrast injection, respectively, for GD-BOPTA (18). Corresponding values for Gd-DTPA were 325±60 and 555±108 ms. The average value of 547 ms found in our study (on average 11.6 min after contrast injection) as well as its standard deviation (130 ms) correspond well to these literature values. Our study is limited as it only includes a small number of subjects and because imaging was performed with a variable wait time after Gd-contrast administration (retrospective collection of the in vivo images).
The T1 weighting of IR-bSSFP imaging has previously been applied by Detsky et al. in myocardial viability imaging for visualizing myocardial infarction in a technique termed multi-contrast late enhancement (MCLE) (19). To that purpose, a segmented IR-bSSFP sequence was used in 11 patients. IR-bSSFP images yielded infarct sizes and left ventricular ejection fractions similar to those obtained with IR GRE and standard SSFP, but provided improved visualization of the infarct-blood border as signal from healthy myocardium and blood could be nulled simultaneously (19-21). Further application of this technique was then reported by the same group for investigating papillary muscle involvement in myocardial infarction (21).
Recently, an IR-bSSFP technique was applied by de Sousa et al. to monitor the effect of gadolinium-based contrast injection, post-arterial occlusion reactive hyperemia and exercise on T1, T2 and proton density in calf muscle. The authors of this study conclude that IR-bSSFP offers a temporal resolution adequate to track rapid physiological adaptations in skeletal muscle (22).
Other techniques, such as modified Look Locker inversion recovery (MOLLI) (23) exist and have been demonstrated to yield single-slice T1 maps of the myocardium by merging images from three consecutive IR experiments. In contrast to the method presented here, it was shown to exhibit dependence on heart rates for long T1 values and is currently not available as a clinical product sequence from all vendors. Piechnik et al. have recently presented a method called Shortened Modified Look-Locker Inversion Recovery (ShMOLLI) that overcomes many of these limitations (24). In contrast to the MOLLI sequence, in the here presented approach, the analyzed image data is acquired during the entire cardiac cycle, not only during diastole where the heart is mainly at rest. As a consequence, both T1 and T2 values are determined for a ROI defined by the user at every time point. While this precludes the creation of pixel maps for T1 and T2, a dynamic sequence of images during the entire cardiac cycle is obtained which can be used to assess cardiac function together with relaxation behavior of the magnetization. ROI analysis is useful for global cardiac diseases including the group of metabolic diseases like amyloid heart disease, Fabry disease but also including the big group of cardiomyopathies like dilatative, concentric and restrictive cardiomyopathy. Furthermore ischemic diseases can show a global pattern in particular when advanced heart failure occurs.
The good agreement of the measured values with the spin-system simulations confirms the applicability of Eqs. [1-7]. In a further study, these simulations could be used to investigate limitations of the method, such as B1 inhomogeneity, off-resonance effects or the influence of cardiac arrhythmia.
As applied here, limitations of the IR-bSSFP technique are in the relatively short wait time (2,000 ms) for the recovery of the longitudinal magnetization. However, for in vitro T1 values in the same range as for in vivo T1 values after contrast injection (i.e., smaller than 800 ms, see Tables 2,3), the method described here agreed favorably with the TSE measurements. Existing deviations may be due to the relatively short recovery time for the longitudinal magnetization (two seconds) relative to the T1 values. Another limitation of our study is the variation in the delay time from contrast administration to image acquisition (7 to 22 min). Patient-specific variations of this delay time are caused by variability in the clinical MRI protocol. To investigate the effect of the delay time on the measurement values, T1 and T2 values could be acquired at different time points after contrast administration. Deviations in T2 might be expected from contributions of a range of effective flip angles across the slice profile. Cooper et al. recently reported a flip angle correction method for T1 and T2 quantification with MOLLI 2D steady-state free precession imaging (25). In the equation approximating the recovery of the longitudinal IR-bSSFP magnetization Eq. [1], the target flip angle value from the scan protocol (50 degrees) was used rather than attempting to calculate an effective flip angle based on the distribution function across the slice profile. These deviations might become a concern if the technique presented here is applied for imaging other tissues with higher T1 values (for example brain tissues). Further sources of error might include through-slice motion, magnetization transfer and finite RF effects. A detailed discussion of these effects is out of the scope of this work, the interested reader is referred to Bieri et al. (26) for a more detailed discussion.
Conclusions
A clinically applicable imaging technique based on cardiac-gated segmented IR-bSSFP was shown to allow quantification of T1 and T2 relaxation times in the myocardium in vivo with an acquisition time in the range of a single breathhold. Average T1 and T2 values compared favorably with literature values. In vitro validation showed good agreement with spin echo methods.
Acknowledgements
Ethical approval: The here presented work has been approved by the Institutional Review Board of the Methodist Hospital and the subjects gave informed consent to the work.
Disclosure: The authors have no conflict of interest except Dr. Peter Schmitt, who is an employee of the Siemens AG.
References
- Lee VS, Resnick D, Bundy JM, et al. Cardiac function: MR evaluation in one breath hold with real-time true fast imaging with steady-state precession. Radiology 2002;222:835-42. [PubMed]
- Carr JC, Simonetti O, Bundy J, et al. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology 2001;219:828-34. [PubMed]
- Pereles FS, Kapoor V, Carr JC, et al. Usefulness of segmented trueFISP cardiac pulse sequence in evaluation of congenital and acquired adult cardiac abnormalities. AJR Am J Roentgenol 2001;177:1155-60. [PubMed]
- Duerk JL, Lewin JS, Wendt M, et al. Remember true FISP? A high SNR, near 1-second imaging method for T2-like contrast in interventional MRI at .2 T. J Magn Reson Imaging 1998;8:203-8. [PubMed]
- Deimling M, Heid O. Magnetization-prepared TrueFISP imaging. Proceedings of SMR 1994.
- Nishimura DG, Vasanawala S. Analysis and reduction of the transient response in SSFP imaging. Proceedings of the 8th Annual Meeting of ISMRM 2000.
- Schmitt P, Griswold MA, Jakob PM, et al. Inversion recovery TrueFISP: quantification of T(1), T(2), and spin density. Magn Reson Med 2004;51:661-7. [PubMed]
- Gulani V, Schmitt P, Griswold MA, et al. Towards a single-sequence neurologic magnetic resonance imaging examination: multiple-contrast images from an IR TrueFISP experiment. Invest Radiol 2004;39:767-74. [PubMed]
- Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 2006;118:10-24. [PubMed]
- Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006;48:1977-85. [PubMed]
- Bankamp A, Schad LR. Comparison of TSE, TGSE, and CPMG measurement techniques for MR polymer gel dosimetry. Magn Reson Imaging 2003;21:929-39. [PubMed]
- Berstein MA, King KF, Zhou XJ. eds. Handbook of MRI Pulse Sequences. Elsevier, Academic Press, 2004.
- Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med 1995;34:910-4. [PubMed]
- Markl M, Alley MT, Elkins CJ, et al. Flow effects in balanced steady state free precession imaging. Magn Reson Med 2003;50:892-903. [PubMed]
- Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol 2008;52:1574-80. [PubMed]
- Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009;11:56. [PubMed]
- Verhaert D, Thavendiranathan P, Giri S, et al. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging 2011;4:269-78. [PubMed]
- Krombach GA, Hahnen C, Lodemann KP, et al. Gd-BOPTA for assessment of myocardial viability on MRI: changes of T1 value and their impact on delayed enhancement. Eur Radiol 2009;19:2136-46. [PubMed]
- Detsky JS, Stainsby JA, Vijayaraghavan R, et al. Inversion-recovery-prepared SSFP for cardiac-phase-resolved delayed-enhancement MRI. Magn Reson Med 2007;58:365-72. [PubMed]
- Connelly KA, Detsky JS, Graham JJ, et al. Multicontrast late gadolinium enhancement imaging enables viability and wall motion assessment in a single acquisition with reduced scan times. J Magn Reson Imaging 2009;30:771-7. [PubMed]
- Yang Y, Connelly K, Graham JJ, et al. Papillary muscle involvement in myocardial infarction: initial results using multicontrast late-enhancement MRI. J Magn Reson Imaging 2011;33:211-6. [PubMed]
- de Sousa PL, Vignaud A, Fleury S, et al. Fast monitoring of T(1), T(2), and relative proton density (M(0)) changes in skeletal muscles using an IR-TrueFISP sequence. J Magn Reson Imaging 2011;33:921-30. [PubMed]
- Messroghli DR, Radjenovic A, Kozerke S, et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 2004;52:141-6. [PubMed]
- Piechnik SK, Ferreira VM, Dall’Armellina E, et al. Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [PubMed]
- Cooper MA, Nguyen TD, Spincemaille P, et al. Flip angle profile correction for T1 and T2 quantification with look-locker inversion recovery 2D steady-state free precession imaging. Magn Reson Med 2012;68:1579-85. [PubMed]
- Bieri O, Scheffler K. On the origin of apparent low tissue signals in balanced SSFP. Magn Reson Med 2006;56:1067-74. [PubMed]


