Aortic regurgitation after transcatheter aortic valve implantation: mechanisms and implications
Introduction
Degenerative aortic stenosis (AS) is the most common isolated valve disease in the Western world (1,2), and its prevalence is steadily increasing with the ageing population (3). Surgical aortic valve replacement (SAVR) remains the standard treatment for patients with severe symptomatic AS (4). Over the last decade, however, transcatheter aortic valve implantation (TAVI) has emerged as a less invasive treatment option for patients with contraindications to open heart surgery, high surgical risk, severe comorbidities, old age, or reduced left ventricular ejection fraction (5-8). This novel technique is now performed with high procedural success. Superiority to medical management as well as non-inferiority to high-risk surgery have been demonstrated in randomized clinical trials (6,9-11). The Edwards SAPIEN and the Medtronic CoreValve prosthesis currently are the two most widely used prostheses worldwide. Procedural complications such as major bleeding, vascular complications, stroke, and death are dreaded in the context of transcatheter valve implantation (6). Improvements of screening standards, implantation techniques, and post-procedural intensive care have considerably reduced complication rates in recent years (12-14).
Aortic regurgitation (AR), predominantly of the paravalvular type, is considered the most common and characteristic drawback of transcatheter valves (15). The aim of this review is to give an overview of the different types of AR occurring after transcatheter valve implantation with a focus on the pathophysiological mechanisms.
Prevalence of aortic regurgitation in transcatheter valves
Hemodynamic results of transcatheter valves successfully implanted within a degenerated native aortic valve are excellent with regard to post-procedural transvalvular pressure gradients. Mean transvalvular pressure gradients and valve effective orifice areas are at least comparable to surgically implanted prostheses (9,16). However, AR is more frequently observed after TAVI as compared to SAVR. In the PARTNER trial, moderate to severe AR was observed in 6.8% of patients in the TAVI group compared to 1.9% in the surgically treated group at 1 year follow-up (9). Some degree of AR, mostly mild, has been reported in about 70% of TAVI patients after up to 1 year follow-up; moderate to severe forms have been documented in 4% to 35% of patients in different studies (9,17-21). In the U.K. TAVI Registry including 870 patients, some degree of paravalvular AR was observed in 61% of patients, being moderate to severe in 13.6% (21). In the European Sentinel Registry of Transcatheter Aortic Valve Implantation including over 4,500 patients, grade 2 AR was observed in 7.7% and grade 3 in 1.3% in the pre-discharge echocardiography study (22). In patients treated with transcatheter valve-in-valve implantation for failed surgical bioprostheses, the incidence of significant AR seems to be comparable to TAVI in native valves; however, increased transvalvular pressure gradients are a major concern in these patients. In the Global Valve-in-Valve Registry, 5% of patients had ≥+2 degree of AR after the procedure (23).
Whereas long-term follow-up is available for surgically implanted bioprostheses, experience is limited to a few years in TAVI patients (24,25). In patients with a Toronto stentless porcine valve, freedom from significant AR was reported to be about 97% at 5 years and 83% at 9 years, respectively (26). Similarly, after aortic valve replacement with a Freestyle bioprosthesis, freedom from significant AR at 15 years was reported to be about 80% (27). So far, both incidence and severity of AR after TAVI are considered to remain stable over time (6,9,19).
Clinical relevance of aortic regurgitation in transcatheter valves
Post-procedural AR has been identified as a strong and independent predictor of all-cause and cardiovascular mortality after TAVI (18,28,29). There is growing evidence that not only moderate to severe AR, but also mild forms impair patient outcome after TAVI, and that the prognostic implications of AR have been underestimated so far (11,18). Indeed, an increased in-hospital mortality of over 12% has been observed in patients with grade 2/4 to 4/4 AR compared to less than 8% in those with grade 1/4 or no AR (18). In the 2-year follow-up of the PARTNER trial, the effect of AR on mortality was proportional to the severity of the regurgitation (11). Furthermore, the presence of AR affects heart failure symptoms after TAVI, since 80% of patients with none or mild AR improved their New York Heart Association (NYHA) functional class after TAVI compared to 60% of patients with moderate to severe regurgitation (30). The causal relationship between mild AR and increased mortality after TAVI as well as the pathophysiological mechanisms underlying this observation remain to be elucidated. This is particularly important because the regurgitation severity is difficult to assess by transthoracic echocardiography, mainly in elderly patients after TAVI, and might easily be underestimated at least in some patients.
The discrepancies in the reported prevalence and severity of AR after TAVI might at least in part be due to the absence of standardized definitions and specific protocols to detect and score AR in transcatheter valves. According to the Valve Academic Research Consortium (VARC) consensus document, the standard classification used to describe regurgitation in native valves has been adopted to TAVI patients (31). However, a comprehensive and quantitative echocardiographic evaluation of AR in prosthetic valves is challenging, in particular in transcatheter valves (32). Multiple jets, eccentric jets, and different types of regurgitation may co-exist in one patient, and anteriorly located jets may be hidden in transesophageal echocardiography by the stent-induced shadowing artifacts. Similarly, two-dimensional transthoracic echocardiography is considered to underestimate the degree of AR after TAVI, in particular with the eccentric or irregularly shaped jets often encountered with paravalvular leaks. To improve the echocardiographic evaluation of TAVI patients, determination of vena contracta planimetry has been recommended (33). Furthermore, integrative approaches such as calculation of regurgitation fraction based on stroke volumes measured by three-dimensional transthoracic echocardiography have been suggested. The latter provides more information regarding both location and extent of paravalvular regurgitation because the regurgitation jet can better be visualized (34-36). Cardiac magnetic resonance imaging may further add to a detailed analysis of regurgitation after TAVI (37), in particular in the assessment of selected patients with significant or ill-defined AR. However, due to its easy applicability and low cost, echocardiography remains the method of choice in the assessment of valvular regurgitation after TAVI.
Despite the high incidence of mild AR following TAVI, specific evaluation criteria for the assessment of AR are lacking, and pathophysiological mechanisms are controversially discussed. A better understanding of regurgitation mechanisms may lead to improvements in both implantation technique and prosthesis design and thereby further optimize the outcome after TAVI.
Different types of transcatheter aortic valve regurgitation
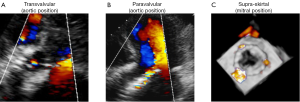
Consistent with the VARC consensus documents and according to current echocardiographic guidelines, regurgitation after TAVI is graded as mild, moderate, or severe (31,38). Traditionally, regurgitation is categorized as transvalvular (located within the prosthesis), paravalvular (located between the prosthesis and the native aortic annulus), or combined (Figures 1,2). A third form of regurgitation termed supra-skirtal has recently been described (Figures 1C,2C) (39). To understand the different mechanisms of AR in transcatheter valves, some anatomic principles and procedural characteristics need to be reflected.
The aortic annulus as the landing zone of the implanted prosthesis represents the most relevant anatomic structure with regard to the development of post-procedural AR (15,40). The aortic root forms the continuation of the left ventricular outflow tract and consists of the aortic annulus, the leaflets of the aortic valve, the fibrous interleaflet triangles, and the sinus of Valsalva (40). The leaflets themselves are attached in a crown-like fashion and show a considerable intra- and interindividual variability in size and shape (41). Hence, a detailed understanding of the individual aortic root anatomy including the relation to coronary artery ostia as well as the location and extent of calcifications is important (40). In contrast to surgery, direct inspection of the aortic root and sizing of the aortic annulus is not possible during TAVI, and only indirect assessment utilizing multimodality imaging with three-dimensional transesophageal echocardiography, multislice computed tomography (MSCT), and angiography is possible (42). In most centers, measurements of the aortic annulus mainly rely on MSCT measurements (43,44).
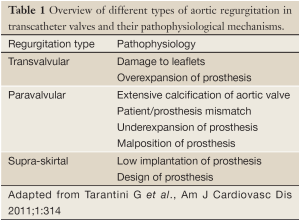
Paravalvular AR is the result of incomplete apposition of the prosthesis to the aortic annulus (Figures 1B,2B). It seems conceivable that calcifications of the aortic annulus mainly promote post-procedural paravalvular AR, since the implanted prosthesis is anchored within the annulus and complete device expansion may be hindered leading to paravalvular residual spaces (45). Indeed, calcifications of the aortic annulus including those of the sinuses of Valsalva as well as those at the commissures were associated with post-procedural paravalvular AR (45-48). In contrast, an association of aortic leaflet calcifications and post-procedural AR has been discussed controversially. While some studies showed an association of leaflet calcifications and regurgitation severity after TAVI (17,48,49), other studies did not (18,42,50). In the prospective multicenter German TAVI registry including over 1,300 patients, no differences were observed between patients with mild, moderate, and severe aortic valve calcification regarding the severity of residual AR immediately after the procedure (50). Besides extensive calcification of the aortic annulus, device-annulus mismatch due to undersizing or underdilatation of the prosthesis as well as malposition of the prosthesis are considered to be among the main risk factors for post-procedural paravalvular AR (15,41,51,52). Hence, precise pre-procedural assessment of aortic annulus dimensions and calcifications is of major importance in these patients. Echocardiographic measurements alone may underestimate aortic annulus dimensions as compared with MSCT, suggesting that both imaging modalities should be considered in the pre-interventional assessment of TAVI patients (36,53-55). Small aortic valve areas and low left ventricular ejection fraction have been associated with an increased incidence of AR, indicating that the pre-interventional imaging should be performed in a particularly careful manner in these patients (17,18,35,48,56,57). In contrast to the above mentioned parameters, no correlation with regurgitation severity was observed for baseline left ventricular outflow tract and aortic root dimensions, mean transvalvular pressure gradients, pre-procedural aortic or mitral regurgitation, and prosthesis size (17,18). Some studies reported a higher prevalence of moderate to severe AR in patients who received a CoreValve prosthesis (21,22,58), while others failed to show any differences between the two most commonly used prosthesis types (18,59).
Transvalvular AR is the result of restricted leaflet motion, leaflet destruction occurring during crimping or implantation, and incorrect sizing or overdilatation of the valve (Figures 1A,2A) (52). Furthermore, larger aortic annulus dimensions have been proposed as a predictor of post-procedural transvalvular AR since more extensive post-dilatation may cause central leaflet separation in those patients (17).
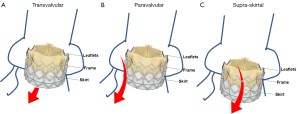
A third type of AR has been proposed in some case reports and comments (Figures 1C,2C) (12,18,39,52). In the two most widely used prostheses, the Edwards SAPIEN and the Medtronic CoreValve prostheses, only the lower part of the frame is covered by a skirt while the upper part is left uncovered (60,61). Hence, leakage through the uncovered part of the prosthesis above the skirt may occur if the prosthesis is implanted too low in the aortic postition. Therefore, we proposed to name this type of regurgitation supra-skirtal regurgitation (39). Consistent with this interpretation, implantation of a balloon-expandable valve at a higher than usual position was found to be effective for preventing regurgitation after TAVI (47). Since the commissures cover a larger part of the steel frame of the prosthesis, regurgitation jets are located in between the commissures and positioned approximately 120° apart from each other when a supra-skirtal regurgitation occurs. This phenomenon was described in a patient with an Edwards SAPIEN prosthesis implanted in mitral position, as the regurgitation jets could be easily visualized in the left atrium in this particular setting (Figure 2C) (39). Because the left ventricular outflow tract is narrow, clear delineation of the jets is difficult in prostheses implanted in aortic position. Accordingly, a substantial number of AR classified as paravalvular might indeed be supra-skirtal.
Hence, patient-, device-, and procedure-related factors contribute to the pathophysiology of AR after TAVI (Table 1). Classification of the different types of AR and identification of the underlying factors are important to reduce the incidence of AR in transcatheter valves. While extensive calcification of the aortic annulus might primarily cause paravalvular AR, too low implantated prosthesis might primarily cause supra-skirtal AR. Hence, the risk of post-procedural AR can at least in part be anticipated from pre-interventional imaging of the aortic root; and accurate pre-procedural assessment of the aortic valvar complex allows to correctly size and position the prosthesis in order to minimize post-procedural AR (40).
Full Table
Conclusions
AR is common and mostly mild after TAVI. Besides device annulus mismatch and suboptimal positioning of the prosthesis, calcifications of the aortic annulus are among the most important predictors of post-procedural AR. We suggest to classify AR in transcatheter valves into three pathophysiological types: (I) paravalvular, (II) transvalvular, and (III) supra-skirtal regurgitation. The importance of multimodality imaging in the assessment of these patients can not be emphasized enough, since a deeper understanding of regurgitation mechanisms and aortic root anatomy in its entirety may improve device design, implantation techniques, and in turn patient outcome.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11.
- Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol 2012;60:1854-63.
- Lindroos M, Kupari M, Heikkila J, et al. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993;21:1220-5.
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-44.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006-8.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607.
- Stähli BE, Bünzli R, Grünenfelder J, et al. Transcatheter aortic valve implantation (TAVI) outcome according to standardized endpoint definitions by the Valve Academic Research Consortium (VARC). J Invasive Cardiol 2011;23:307-12.
- Ozkan A. Low gradient “severe” aortic stenosis with preserved left ventricular ejection fraction. Cardiovasc Diagn Ther 2012;2:19-27.
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696-704.
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686-95.
- Buellesfeld L, Grube E. A permanent solution for a temporary problem: transcatheter valve-in-valve implantation for failed transcatheter aortic valve replacement. JACC Cardiovasc Interv 2012;5:578-81.
- Webb JG, Altwegg L, Boone RH, et al. Transcatheter aortic valve implantation: impact on clinical and valve-related outcomes. Circulation 2009;119:3009-16.
- Gurvitch R, Tay EL, Wijesinghe N, et al. Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv 2011;78:977-84.
- Gotzmann M, Lindstaedt M, Mugge A. From pressure overload to volume overload: aortic regurgitation after transcatheter aortic valve implantation. Am Heart J 2012;163:903-11.
- Zahn R, Gerckens U, Grube E, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J 2011;32:198-204.
- Yared K, Garcia-Camarero T, Fernandez-Friera L, et al. Impact of aortic regurgitation after transcatheter aortic valve implantation: results from the REVIVAL trial. JACC Cardiovasc Imaging 2012;5:469-77.
- Abdel-Wahab M, Zahn R, Horack M, et al. Aortic regurgitation after transcatheter aortic valve implantation: incidence and early outcome. Results from the German transcatheter aortic valve interventions registry. Heart 2011;97:899-906.
- Rajani R, Kakad M, Khawaja MZ, et al. Paravalvular regurgitation one year after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2010;75:868-72.
- Yan TD, Cao C, Martens-Nielsen J, et al. Transcatheter aortic valve implantation for high-risk patients with severe aortic stenosis: A systematic review. J Thorac Cardiovasc Surg 2010;139:1519-28.
- Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130-8.
- Di Mario C, Eltchaninoff H, Moat N, et al. The 2011-12 pilot European Sentinel Registry of Transcatheter Aortic Valve Implantation: in-hospital results in 4,571 patients. EuroIntervention 2012. [Epub ahead of print].
- Dvir D, Webb J, Brecker S, et al. Transcatheter Aortic Valve Replacement for Degenerative Bioprosthetic Surgical Valves: Results From the Global Valve-in-Valve Registry. Circulation 2012;126:2335-44.
- Litzler PY, Borz B, Smail H, et al. Transapical aortic valve implantation in Rouen: four years’ experience with the Edwards transcatheter prosthesis. Arch Cardiovasc Dis 2012;105:141-5.
- Toggweiler S, Humphries KH, Lee M, et al. 5-year outcome after transcatheter aortic valve implantation. J Am Coll Cardiol 2013;61:413-9.
- Bach DS, Goldman B, Verrier E, et al. Durability and prevalence of aortic regurgitation nine years after aortic valve replacement with the Toronto SPV stentless bioprosthesis. J Heart Valve Dis 2004;13:64-72; discussion 72.
- Mohammadi S, Tchana-Sato V, Kalavrouziotis D, et al. Long-term clinical and echocardiographic follow-up of the Freestyle stentless aortic bioprosthesis. Circulation 2012;126:S198-204.
- Gotzmann M, Korten M, Bojara W, et al. Long-term outcome of patients with moderate and severe prosthetic aortic valve regurgitation after transcatheter aortic valve implantation. Am J Cardiol 2012;110:1500-6.
- Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 2012;366:1705-15.
- Nielsen HH, Egeblad H, Andersen HR, et al. Aortic regurgitation after transcatheter aortic valve implantation of the edwards SAPIEN(tm) valve. Scand Cardiovasc J 2013;47:36-41.
- Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18.
- Mohr-Kahaly S, Kupferwasser I, Erbel R, et al. Value and limitations of transesophageal echocardiography in the evaluation of aortic prostheses. J Am Soc Echocardiogr 1993;6:12-20.
- Gonçalves A, Almeria C, Marcos-Alberca P, et al. Three-dimensional echocardiography in paravalvular aortic regurgitation assessment after transcatheter aortic valve implantation. J Am Soc Echocardiogr 2012;25:47-55.
- Anwar AM, Nosir YF, Zainal-Abidin SK, et al. Real-time three-dimensional transthoracic echocardiography in daily practice: initial experience. Cardiovasc Ultrasound 2012;10:14.
- Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25:3-46.
- Thavendiranathan P, Liu S, Datta S, et al. Automated quantification of mitral inflow and aortic outflow stroke volumes by three-dimensional real-time volume color-flow Doppler transthoracic echocardiography: comparison with pulsed-wave Doppler and cardiac magnetic resonance imaging. J Am Soc Echocardiogr 2012;25:56-65.
- Sherif MA, Abdel-Wahab M, Beurich HW, et al. Haemodynamic evaluation of aortic regurgitation after transcatheter aortic valve implantation using cardiovascular magnetic resonance. EuroIntervention 2011;7:57-63.
- Lancellotti P, Tribouilloy C, Hagendorff A, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr 2010;11:223-44.
- Stähli BE, Gebhard C, Falk V, et al. Regurgitation after Edwards SAPIEN valve implantation: truly paravalvular or ‘supra-skirtal’? Eur Heart J 2012. [Epub ahead of print].
- Piazza N, de Jaegere P, Schultz C, et al. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv 2008;1:74-81.
- Silver MA, Roberts WC. Detailed anatomy of the normally functioning aortic valve in hearts of normal and increased weight. Am J Cardiol 1985;55:454-61.
- Wood DA, Tops LF, Mayo JR, et al. Role of multislice computed tomography in transcatheter aortic valve replacement. Am J Cardiol 2009;103:1295-301.
- Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2008;29:1463-70.
- Achenbach S, Delgado V, Hausleiter J, et al. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012;6:366-80.
- Ewe SH, Ng AC, Schuijf JD, et al. Location and severity of aortic valve calcium and implications for aortic regurgitation after transcatheter aortic valve implantation. Am J Cardiol 2011;108:1470-7.
- Gripari P, Ewe SH, Fusini L, et al. Intraoperative 2D and 3D transoesophageal echocardiographic predictors of aortic regurgitation after transcatheter aortic valve implantation. Heart 2012;98:1229-36.
- Unbehaun A, Pasic M, Dreysse S, et al. Transapical aortic valve implantation: incidence and predictors of paravalvular leakage and transvalvular regurgitation in a series of 358 patients. J Am Coll Cardiol 2012;59:211-21.
- John D, Buellesfeld L, Yuecel S, et al. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc Interv 2010;3:233-43.
- Koos R, Mahnken AH, Dohmen G, et al. Association of aortic valve calcification severity with the degree of aortic regurgitation after transcatheter aortic valve implantation. Int J Cardiol 2011;150:142-5.
- Staubach S, Franke J, Gerckens U, et al. Impact of aortic valve calcification on the outcome of transcatheter aortic valve implantation: Results from the prospective multicenter German TAVI registry. Catheter Cardiovasc Interv 2013;81:348-55.
- Ussia GP, Barbanti M, Imme S, et al. Management of implant failure during transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2010;76:440-9.
- Tarantini G, Gasparetto V, Napodano M, et al. Valvular leak after transcatheter aortic valve implantation: a clinician update on epidemiology, pathophysiology and clinical implications. Am J Cardiovasc Dis 2011;1:312-20.
- Ng AC, Delgado V, van der Kley F, et al. Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ Cardiovasc Imaging 2010;3:94-102.
- Gao G, Wu Y, Grunkemeier GL, et al. Durability of pericardial versus porcine aortic valves. J Am Coll Cardiol 2004;44:384-8.
- Tops LF, Wood DA, Delgado V, et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging 2008;1:321-30.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 2010;121:1848-57.
- Clavel MA, Webb JG, Pibarot P, et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol 2009;53:1883-91.
- Nombela-Franco L, Ruel M, Radhakrishnan S, et al. Comparison of Hemodynamic Performance of Self-Expandable CoreValve Versus Balloon-Expandable Edwards SAPIEN Aortic Valves Inserted by Catheter for Aortic Stenosis. Am J Cardiol 2013. [Epub ahead of print].
- Chieffo A, Buchanan GL, Van Mieghem NM, et al. Transcatheter Aortic Valve Implantation With the Edwards SAPIEN Versus the Medtronic CoreValve Revalving System Devices: A Multicenter Collaborative Study: The PRAGMATIC Plus Initiative (Pooled-RotterdAm-Milano-Toulouse In Collaboration). J Am Coll Cardiol 2013. [Epub ahead of print].
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007;116:755-63.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007;50:69-76.


